Global Distribution of Omura’s Whales (Balaenoptera omurai) and Assessment of Range-Wide Threats
- 1Anderson Cabot Center for Ocean Life, New England Aquarium, Boston, MA, United States
- 2National Museum of Nature and Science, Tokyo, Japan
- 3Southwest Fisheries Science Center, NOAA Fisheries, Monterey, CA, United States
When the Omura’s whale (Balaenoptera omurai) was first described in 2003, it was known from only three locations: the southern Sea of Japan, and the vicinities of the Cocos (Keeling) Islands and Solomon Islands. Work over the following decade suggested a range limited to the eastern Indo-Pacific, but more recent discoveries in the western Indian Ocean and Atlantic Ocean suggested a more widespread range than previously thought. Here we use all available sources of information, including published papers, unpublished reports, and internet-based accounts, substantiated through genetic, morphological, photographic and acoustic documentation, to compile accounts of Omura’s whales globally. Reports increased precipitously since 2015 after publication of the first detailed external description of the species, reflecting the impact of the recently elevated awareness of the species. We report 161 accounts from 95 locales in the waters of 21 range states, and found that the species is widely distributed in primarily tropical and warm-temperate locations. Currently it is known from all ocean basins with the exception of the central and eastern Pacific. The majority of accounts remain in the eastern Indo-Pacific suggesting a potentially recent range expansion from this region. There is a strong tendency toward a coastal and neritic distribution, although there are also several pelagic records. A predominantly near-coastal distribution places Omura’s whale at risk from anthropogenic activities throughout its range, and its tropical distribution in often remote and poorly monitored areas makes adequately documenting and assessing threats challenging. We assess documented threats in light of the reported species’ range, and found threats from, at minimum, ship strikes, fisheries bycatch and entanglement, local directed hunting, petroleum exploration (seismic surveys), and coastal industrial development. Current evidence indicates that at least some populations are non-migratory with local, potentially restricted ranges. Furthermore, there is low genetic diversity documented throughout its global distribution. Given the species may be characterized by small local populations, it may be particularly vulnerable to impacts from existing regional anthropogenic threats. We recommend that focused work be conducted to locate and study local populations, assess potential population isolation, and determine conservation status and specific anthropogenic threats across the species’ range.
Introduction
The Omura’s whale (Balaenoptera omurai) was described as a new species distinct from and sister to the clade formed by the Bryde’s whales (B. edeni brydei and B. e. edeni) and sei whale (B. borealis), through mitochondrial DNA sequencing, skeletal morphology and a limited description of external characteristics (Wada et al., 2003). A species-level status was first proposed by Wada and Numachi (1991) and Yoshida and Kato (1999), and the phylogenetic relationship supported by work using additional markers (Sasaki et al., 2006; Rosel and Wilcox, 2014). Estimated time of lineage divergence was initially 17 million years (Sasaki et al., 2006), but more recent work suggests a more recent divergence at 7–9 million years (Marx and Fordyce, 2015; Slater et al., 2017). The initial species description was based on a 1998 stranding in the Sea of Japan (the holotype specimen, which included the complete skeleton and baleen plates) along with tissues, morphological data and descriptions of eight specimens taken in the late 1970s by Japanese research whaling vessels near the Cocos (Keeling) Islands and Solomon Islands (Wada et al., 2003). Prior to the collection of the holotype specimen, the eight whaling specimens had been attributed to Bryde’s whale (B. edeni) but recognized as distinct due to their diminutive size, divergent allozyme profiles and partial mitochondrial DNA (mtDNA) haplotypes; these were referred to as “small” or “small-form” Bryde’s whales (Ohsumi, 1980; Wada and Numachi, 1991; Yoshida and Kato, 1999). Relatively concurrent with work on these aforementioned specimens, indigenous whaling specimens from the Bohol Sea, Philippines, were noted to have atypically small skulls for B. edeni and divergent mtDNA cytochrome b sequences (Perrin et al., 1996; LeDuc and Dizon, 2002) and were later identified as belonging to B. omurai (Sasaki et al., 2006; Yamada et al., 2008). Between 2003 and 2012, several specimens were identified primarily through examination of skulls throughout the Southeast Asian region (Yamada et al., 2006a, 2008; Yamada, 2009), which was possible due to the detailed description of skull morphology available from Wada et al. (2003). Through this work, the range of the species was thought to be restricted to the eastern Indo-Pacific region, more specifically the tropical eastern Indian Ocean and western Pacific Ocean.
In 2013 a population of Omura’s whales tractable for long-term monitoring was discovered off the northwest coast of Madagascar, extending the range into the western Indian Ocean (Cerchio et al., 2015). Following shortly on this report, a stranding off the coast of Mauritania was genetically identified extending the range much further into the tropical eastern North Atlantic Ocean (Jung et al., 2015). Prior to 2015 there was only a limited description of the species drawing similarities to the asymmetrical pigmentation of fin whales, based on the Sea of Japan stranding and Cocos (Keeling) Islands and Solomon Islands whaling specimens (Wada et al., 2003); however, no good photographic evidence or any documentation of free-ranging live animals with confirmed species attribution were available. Through the use of detailed surface and underwater still photographs and video, combined with genetic verification (mtDNA) of simultaneously collected skin biopsies, Cerchio et al. (2015) described in detail the external physical appearance and pigmentation patterns. Thereafter, numerous reports have been made in peer-reviewed scientific literature, gray literature and internet-based accounts, primarily in shallow coastal waters and expanding the range to most ocean basins from the western South Atlantic Ocean to the Indian Ocean, the northwest Pacific Ocean and Oceania (Cerchio and Yamada, 2018).
Here we summarize all records that we could find relating to the global distribution of Omura’s whales, drawing upon all available sources of information, in an effort to better establish the known range and begin to assess range-wide threats to the species, especially at the regional level.
Materials and Methods
We use documentation from published papers, unpublished reports, and internet-based accounts, each verified by the authors, to compile available accounts of Omura’s whale as of 2018. Verification of accounts is here reported in a descending hierarchy of methods for diagnosing the species, with the highest level reported when multiple verifications are available for a single account:
(1) Genetic – through sequencing of mtDNA control region, cytochrome b, and/or cytochrome c oxidase subunit 1 markers, as first documented by Wada et al. (2003) reporting the mtDNA control region and Sasaki et al. (2006) reporting the whole mtDNA genome;
(2) Skull Morphology – through detailed examination of identifying characters that distinguish Omura’s whale from similar sized congeners, as first described by Wada et al. (2003);
(3) Photographic (including video) – through identification of diagnostic pigmentation and external physical characteristics as first described by Cerchio et al. (2015);
(4) Acoustic – through identification of diagnostic recorded song phrases as first described by Cerchio et al. (2015);
(5) Visual report – through a reported description congruent with external physical appearance (as in Cerchio et al., 2015) when corroborated with an account using a higher verification method in the same general locale.
Given the nearly global range of the species that is established by this review, potential anthropogenic threats are then assessed globally based on expert knowledge of existing known risks and cases of documented interactions in areas where Omura’s whales were reported.
Results
A total of 161 accounts were found spanning 95 locales from 21 different range states. Results of the review are presented by geographical region and country, and accounts are summarized by diagnostic method (Table 1 and Figure 1). A detailed table of all accounts in MS Excel format and a Google Earth KMZ file with positions of all accounts are provided as Supplementary Material.
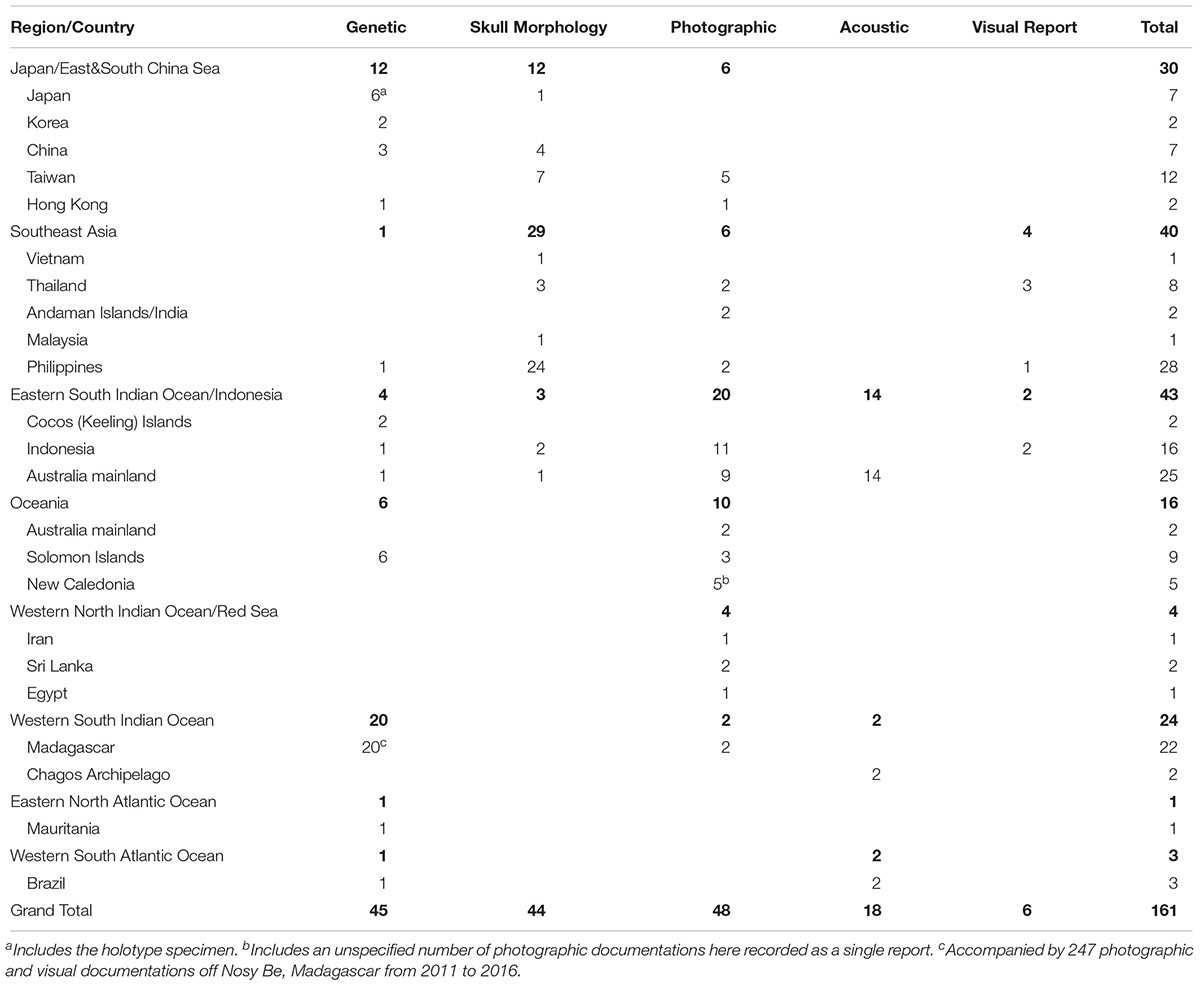
Table 1. Summary of 161 reports of Omura’s whales identified across the range of the species, and verified through one or more of four described methods (Genetic sequencing of mtDNA markers, diagnostic characters of the skull, photographic documentation of diagnostic external characters, and recording of diagnostic vocalization), as well as visual reports with sufficient veracity of identification (accompanied by a previous documentation by another verified methodology in the locale).
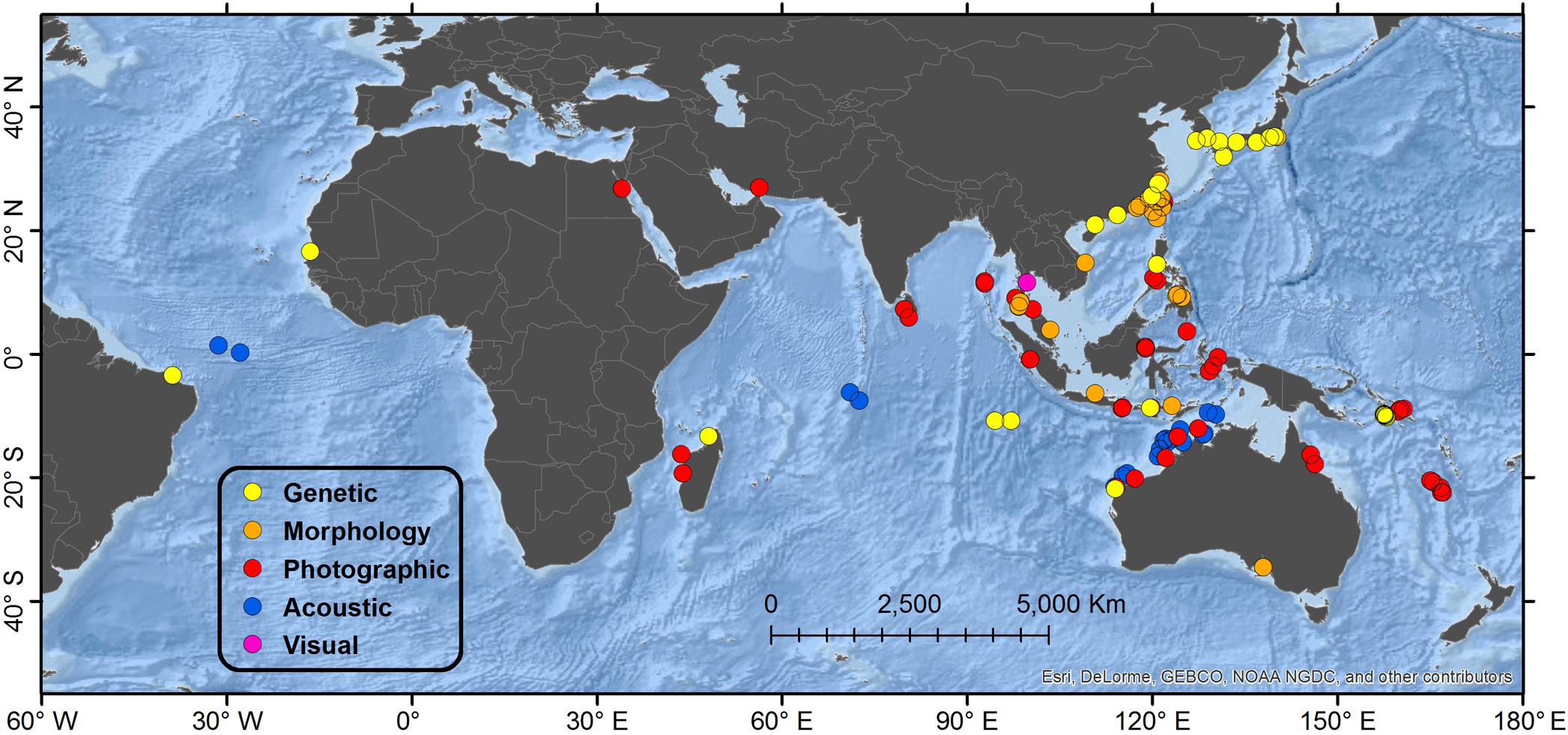
Figure 1. Map showing global distribution of reported Omura’s whales accounts by diagnostic verification method. “Genetic” refers to typing of mtDNA markers; “Morphology” refers specifically to diagnostic characters of the skull; “Photographic” refers to photographs or video illustrating diagnostic external physical characters and pigmentation; “Acoustic” refers to recordings of diagnostic vocalizations; “Visual” refers to a report congruent with external appearance when substantiated by a separate account from the same general locale verified by a higher level method. When multiple methods of verification are available from a single account, the highest level is reported in order of presentation, with “Genetic” being the highest. Details of regions are shown in Figures 2, 3, 5, 6.
Regional Accounts
Japan/East and South China Seas (see Figure 2 for Detail)
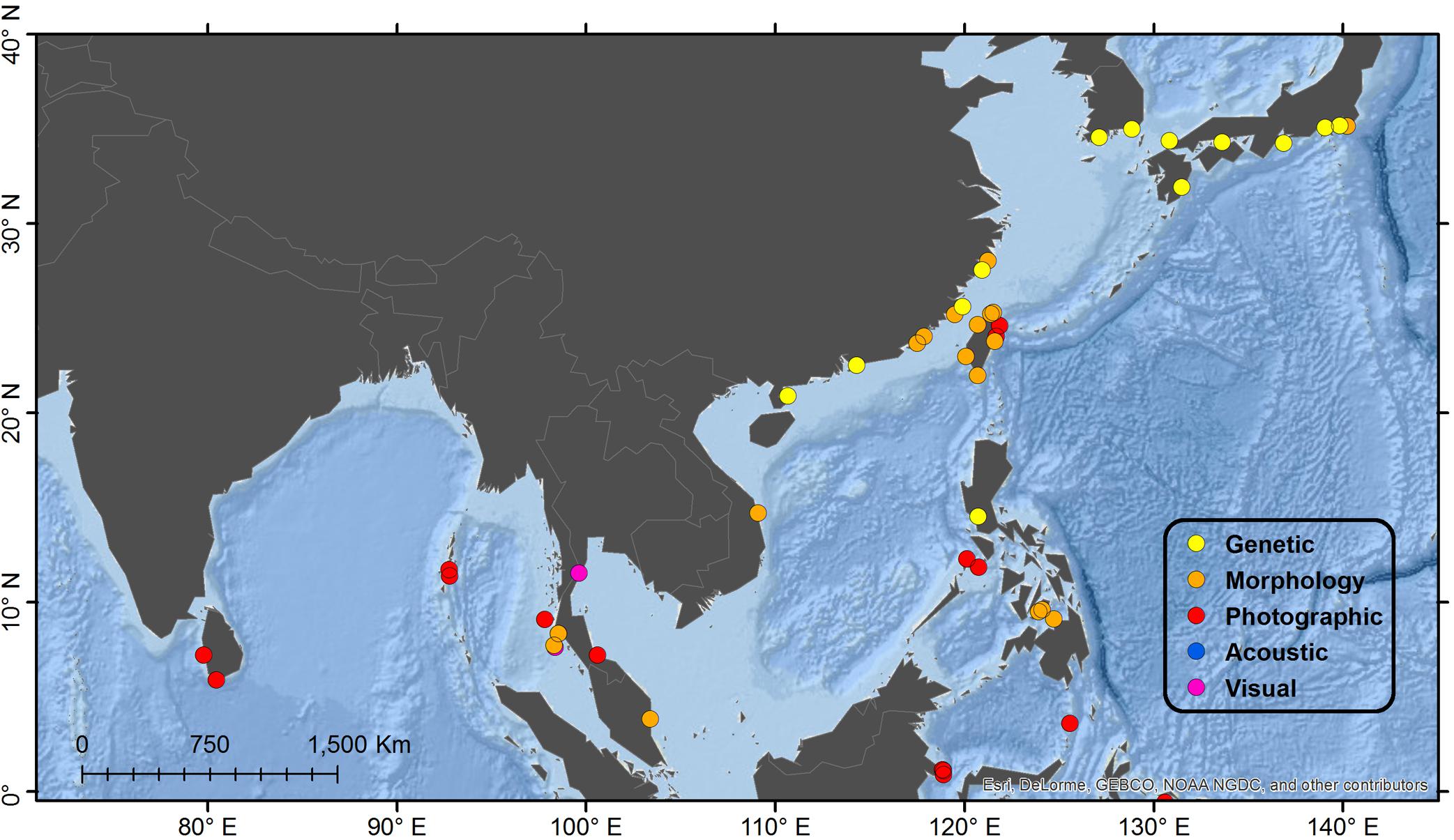
Figure 2. Detail map of northeast Indian Ocean and northwest Pacific Ocean regions. See Figure 1 for description of Legend entries.
Japan
Seven records are known, four strandings and three incidentally bycaught animals. The strandings include the holotype specimen which was a ship strike mortality of an 11 m mature female in the Sea of Japan, Tsunoshima Island in September 1998 (Wada et al., 2003), a record of a 7.1 m female from the Inland Sea, Awashima Island, Kagawa Prefecture in June 2002 (Sasaki et al., 2006), a 3.2 m neonate female in Miyazaki City, Miyazaki Prefecture in August 2005 (Yamada, 2009) and an 8–9 m physically mature individual of unknown sex in Katsuura, Chiba Prefecture in July 2017 which was identified by skull morphology (Miyakawa, 2018). The Tsunoshima Island specimen, in combination with eight specimens previously taken during scientific whaling operations in international waters off the Cocos (Keeling), and off the Solomon Islands (see below) allowed the designation of species level divergence from other baleen whales using a combination of genetic differentiation and morphological characters. The Miyazaki Prefecture specimen indicates calving at least during the Boreal summer (i.e., June–July) as the specimen was a neonate with a body length of 3.2 m. The remaining three accounts were individuals incidentally caught in local fisheries in Sagami Bay in Shizuoka Prefecture (9.2 m male) in October 2003, in Tokyo Bay, Chiba Prefecture (10.05 m female) in May 2004, and near Ise Bay in Mie Prefecture (6.3 m immature female) in March 2012, all genetically identified as B. omurai (Goto, 2012; Ishikawa et al., 2013).
Republic of Korea
There are two recent records reported from Korea, both of which were bycatch in Korean fisheries and genetically identified from archived preserved tissue specimens (Kim et al., 2018). A 6.3 m female was bycaught in coastal Geoje in January 2004 and a 6.4 m male was bycaught in coastal Goheung in December 2006, the sizes of which suggest juvenile animals based upon size ranges of mature individuals reported in Wada et al. (2003) and estimated size at birth of ca. 3.2 m (Yamada, 2009).
People’s Republic of China (PRC)
Seven records are known on mainland China. In the East China Sea, a stranding from Rui’an City, Zhejiang in November 1994, was reported in Yang et al. (2002) as pygmy Bryde’s whale, Balaenoptera edeni, after genetically matching the Yoshida and Kato (1999) specimens from the Solomon Islands. As this report was prior to the designation of Omura’s whale as a distinct species by Wada et al. (2003), it was later recognized (by Rosel and Wilcox, 2014) that the sequences indicate this specimen is B. omurai. An additional record from the East China Sea was a stranding from Yuhuan County, Zhanjiang Province in December 2001, based on skeletal morphology (Wang et al., 2006). Three stranding records in the Taiwan Strait were identified as B. omurai based on skeletal morphology, from Dongshan County in December 1991, Nanri Island in August 2008, and Zhangpu County in November 2009, all in Fujian Province (Wang, 2012). A 2011 stranding in the Taiwan Strait at Pingtan Island, Fujian Province was recently genetically identified as B. omurai (Xu et al., 2017). A specimen in the South China Sea was genetically identified as B. omurai by Ma et al. (2007) from a single vertebra collected by Zhou Kaiya (personal communication to RLB) from Nao Zhou Island, Zhanjiang, Guangdong Province in February 2005.
Taiwan
Yamada et al. (2006a) identified at least 7 different specimens based on examination of skulls at museums in Taiwan, representing strandings around most coastlines of Taiwan. Records with identifying information included a whale of unknown sex from Pingtung County in November 1990, a 5.54 m male from Tainan County in February 1997, a 5.13 m male from Miaoli County in March 1997, an 8–9 m male from Taipei County in May 1997, a 6.54 m male from Taoyuan County in December 1998, and a 5.95 m female from Hualien County in January 2004. Several photographic accounts have recently been reported off the east coast of Taiwan during whale watching trips from Hualien City, Hualien County, with at least five confirmed records during August 2010, April and May 2016, May 2017, and July 2018 (complied by D. Yasutaka Imai1).
Hong Kong (Special Administrative Region of PRC)
There is an historic record of a live stranding in Hong Kong Harbor in April 1955, described in a magazine of the Hong Kong University as an 8.2 m immature male fin whale, Balaenoptera physalus (Anonymous, 1955). Review of photographs and description suggest it was likely B. omurai. The specimen was recently examined by two of us (TKY and RLB) but it was not possible to definitively identify the species because a thick coating of paint on the skeleton obscured the identifying osteological characteristics. There is at least one documented stranding, in Hung Shek Mun, Plover Cove Country Park, Tai Po, in March 2014 which was genetically identified as B. omurai by Southwest Fisheries Science Center (K. Robertson personal communication to RLB).
Southeast Asia (see Figure 2 for Detail)
Vietnam
A complete skeleton of a 10 m balaenopterid found at Pho Thanh tomb, Pho Thanh commune, Duc Pho district, Quang Ngai province in April 2012 was identified as an Omura’s whale by morphological examination (Chien and Quan, 2013), however, the date of death and reason (stranding, bycatch, etc.) was either not known or not reported. Species identification was confirmed by TKY from photographs of the skull.
Thailand
Reports from Thailand come from both sides of the Malay Peninsula, on the east coast in the Gulf of Thailand and the west coast in the Andaman Sea. Yamada et al. (2006a) identified three specimens through examination of skeletons from strandings, all from the west coast; these included an unknown sex animal in November 1983 and a 10 m male in June 1995 from Phuket Province, and a 4.3 m female in December 1999 from Phang-nga Province. Adulyanukosol et al. (2012) in a book on Bryde’s whales of Thailand, report several accounts of Omura’s whales on both the east and west coasts. These include visual reports on the west coast of four feeding animals from Phuket Island/Racha Noi-Racha Yai Islands, and a stranded calf from Bang Pat, Phang-nga Province (which may be the same 4.3 m female from Yamada et al., 2006a). Adulyanukosol et al. (2012) also report two accounts from the Gulf of Thailand, including a visual report of a stranded 3.87 m calf from Thap Sakae, Prachuap Khiri Khan Province, and a 4.4 m male calf bycaught in local fisheries in Songkhla Province in May 2011, accompanied by identifying photographs. There is also a web-based video documentation of an Omura’s whale in the Andaman Sea at Kho Tachai Pinnacle, National Park Similan Islands from February 2013 (B. Milovanovic2). All accounts off the west coast of Thailand are on the eastern side of the Andaman Sea, relatively close to the Thai mainland.
Andaman Islands (India)
There are two modern reports from the Andaman and Nicobar Islands, on the western side of the Andaman Sea. Photographic evidence from May 2015 east of Port Blair, South Andaman Island, of a medium sized balaenopterid was attributed with high confidence to be B. omurai (Marine Mammal Conservation Network of India3); the encounter was described as involving 4–5 animals moving rapidly with unpredictable surfacing behavior (Mankeshwar, Malavi, and Sutaria, personal communication to SC and RLB), a typical behavior pattern of Omura’s whales described off Madagascar (Cerchio et al., 2015). An additional encounter with photographic evidence was documented in April 2018 northeast of Cinque Island in the South Andaman Islands (Marine Mammal Conservation Network of India4; Mankeshwar, Malavi, and Sutaria, personal communication to SC and RLB).
Malaysia
There is at least one account off the east coast of Peninsular Malaysia, from Ponnampalam (2012), who reported an Omura’s whale that stranded at Pahang in 2008. The species identification was confirmed based on the skull morphology (by TKY). It is possible that this is the same specimen as a stranding in October 2008 reported as a 9.14 m Bryde’s whale (World Environment on NBC News5).
Philippines
A traditional local shore-based whaling operation in the Bohol Sea dated at least back to the 19th century, and took what was first thought to be Bryde’s whales into the late 1990’s (Dolar et al., 1994; Acebes, 2014); there was also a brief period of commercial whaling in the 1980’s, however, it is thought that these whalers primarily hunted offshore in international waters (Perrin, 2007; Acebes, 2014). Dolar et al. (1994) first reported on specimens taken in the Bohol Sea from Pamilacan Island, Lila on Bohol Island, and Sagay on Camiguin Island, which were recognized to be atypically small compared to other B. edeni by Perrin et al. (1996). Later LeDuc and Dizon (2002) used a specimen from this same catch in a genetic phylogeny, placing it in the tree congruent with the position of Omura’s whale (as recognized by Sasaki et al., 2006). Yamada et al. (2008) identified 24 of 28 identifiable specimens from this catch definitively as Omura’s whales based on skull morphology (the remaining four were classified as Bryde’s whales). These were the same specimens as examined by Perrin et al. (1996). This is the best documented directed hunt on a population of Omura’s whales. Modern accounts suggest a more widespread distribution in the Philippines outside of the Bohol Sea, including: a photographic account in the northeastern Sulu Sea in 2010 (Dolar, personal communication confirmed by SC); a photographic account off the northwest coast of Busuanga Island, Palawan in Feb 2016 (Dugong Dive Center6); and a ship shrike account in Manila Bay (Dolar, personal communication, genetic identification from Southwest Fisheries Science Center of sample provided by the Philippines Bureau of Fisheries and Aquatic Resources), although the Manila Bay specimen reportedly came in on the bow of a ship so is likely from an unknown location outside of the Bay. Aragones et al. (2010) report two strandings of Omura’s whale in the Philippines between 1998 and 2009, however provide no details on location, date or specimen characteristics.
Eastern South Indian Ocean/Indonesia (see Figure 3 for Detail)
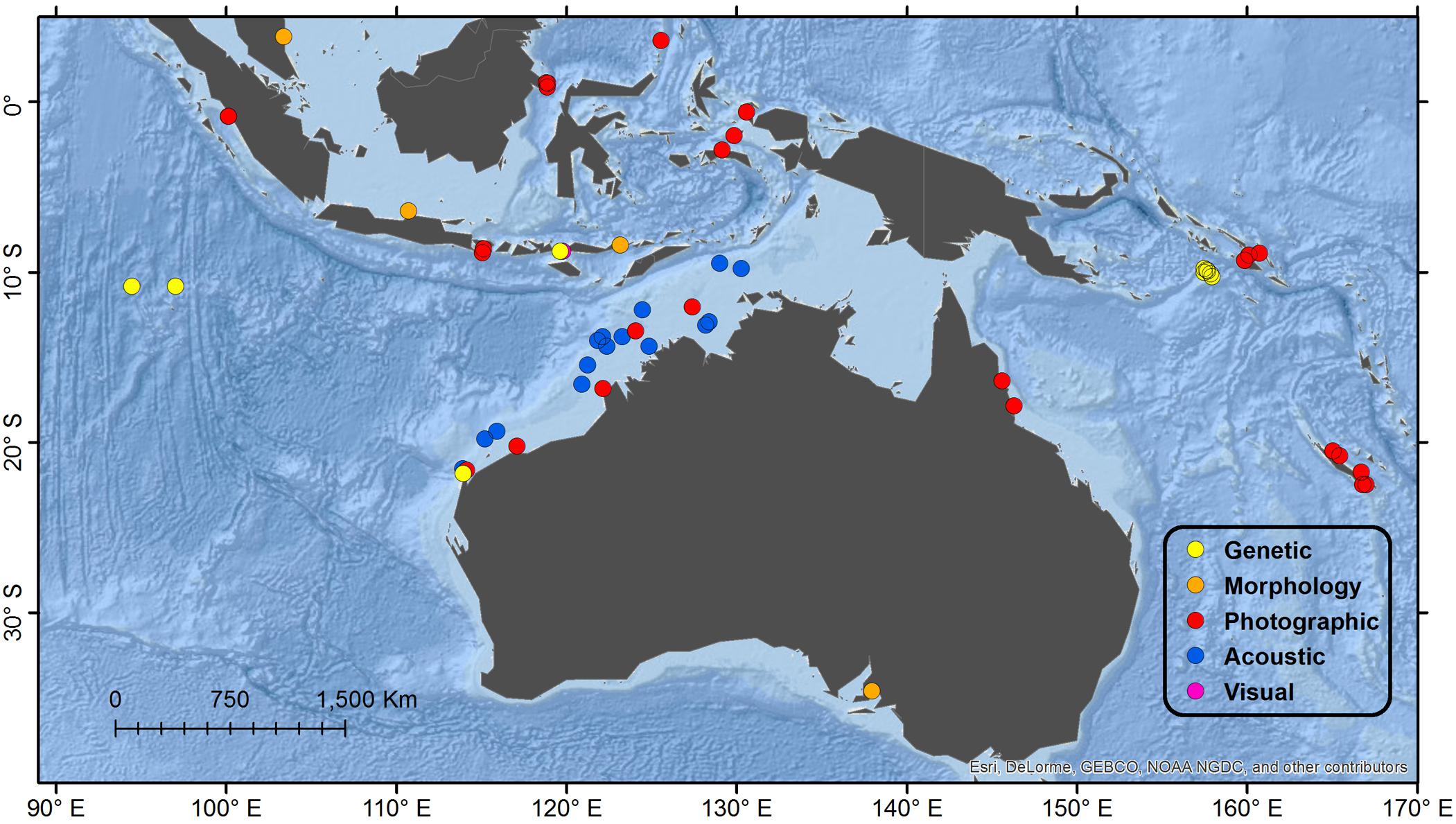
Figure 3. Detail map of southeast Indian Ocean and southwest Pacific Ocean regions. See Figure 1 for description of Legend entries.
Cocos (Keeling) Islands
During whaling operations for Bryde’s whales under scientific permit from 1976 to 1979, Japanese whalers took 459 whales in the Indian and western Pacific Oceans in the Southern Hemisphere (Ohsumi, 1980); 120 specimens were caught in the eastern Indian Ocean, from 92–119°E, 9-14°S during November 1978. Two of these specimens caught ca. 100 km north and 270 km northwest of the Cocos (Keeling) Islands (Indian Ocean Territories of the Commonwealth of Australia) were phylogenetically grouped with the Solomon Islands “small form Bryde’s whale” samples (see below; Wada and Numachi, 1991) and were included among the eight specimens identified as Omura’s whales by Wada et al. (2003). The reported catch positions of these two specimens are unusual in context of most other recorded accounts, in that they are in deep abyssal plain waters > 100 km from the northern most island of the Cocos Archipelago, so far from shallow water habitat. However, Kawamura (1980) indicates that the predominant prey species in 31 of 53 stomachs of the Bryde’s whales caught in this area was the neritic krill species Pseudeuphausia latifrons, a prey item also identified for Omura’s whales in Madagascar (Cerchio et al., 2018; Cerchio and Yamada, 2018). This species of krill is primarily a coastal and continental shelf euphausiid, although it has also been documented in offshore waters (Boden et al., 1955; Brinton, 1975; Wilson et al., 2003). So it is possible that some of these eastern Indian Ocean Bryde’s whales were taken in shallower water than reported, and might include the two later identified as Omura’s whales.
Indonesia
Omura’s whales appear to be widespread in Indonesia with accounts from, moving counter-clockwise from the west, West Sumatra, the south Java Sea, Bali, Komodo, Solor Archipelago, Seram, Raja Ampat, Palau Mansuar, North Sulawesi, and East Kalimantan regions. The first Indonesian accounts appear to be from Weber (1923), who describes three skulls of balaenopterids taken in indigenous hunts; examination of photographs (by TKY) indicated that two of the skulls were Omura’s whales from disparate regions of Indonesia, in Bangsri, Java on the Java Sea, and Lamakera, Solor on the Savu Sea. The first recent accounts come from the Komodo region, with multiple reports of “pygmy Bryde’s whale” (identified at the time as B. edeni) within the Komodo National Park (Kahn, 2001; Kahn et al., 2001). Observations were made during cetacean surveys at Gili Mota in October 1999, at Loh Dasami, Nusa Kode Strait in April and October 2000, and at Loh Laing in October 2000, with a detailed physical description in Kahn (2001) and Kahn et al. (2001) that matches Omura’s whales described in Madagascar (Cerchio et al., 2015). An individual from Loh Dasami was biopsied and genetically identified as “pygmy Bryde’s whale” by Southwest Fisheries Science Center (Kahn, 2001; Kahn et al., 2001), confirming the Omura’s whale attribution. It is worth noting that these Komodo accounts of Kahn and colleagues appear to be the first detailed descriptions globally of living Omura’s whales. The frequency of sightings during cetacean surveys in 1999–2000 (Kahn, 2001) suggests there may exist an accessible resident population in the area (as also occurs off northwest Madagascar, see below). Kahn (2005) during cetacean surveys in the Bali-Lombok Strait region also reported Bryde’s whale at Uluwatu, South Bali, however, identified them as B. brydei despite demonstrating asymmetrical pigmentation in photographs; the photographs confidently indicate it was Omura’s whale. An additional record from the Bali region was identified by review of internet-based stranding records of Whale Stranding Indonesia, with a female neonate stranding at Batu Bolong, Canggu, Kuta Bali in February 2016 (Whale Stranding Indonesia7); this record was identified as B. edeni, but good photographs of the fresh carcass clearly indicate asymmetrical pigmentation and confident attribution of B. omurai. The westernmost records are two recent photographic accounts from West Sumatra (Danielle Kreb, personal communication, Loka KKPN Pekanbaru8, verified by SC) documenting at least three individuals off Palau Pieh and Palau Air, Pandang, including a mother-calf pair, on 2 days in September 2018. There is an account from Ora Beach, North Seram in May 2016, documenting a single feeding individual from drone video footage (Ora Beach Eco Resort, Seram Maluku9). There are at least two records in the Raja Ampat islands, northeast of Seram; on 25 and 26 November 2016 off Misool island, several Omura’s whales were filmed during 2 days, including documentation of feeding and a mother with calf (Alex Lindbloom Photography10, personal communication to SC). The easternmost record is a mother and calf photographically documented in April 2017 in Dampier Strait off Pulau Mansuar, an island just northwest of the Bird’s Head Peninsula, Province of Papua (S. Boonyaguekul11). Off eastern Borneo, there are three photographic accounts from East Kalimantan (Kreb, personal communication, verified by SC): four individuals were sighted off Sandaran, East Kutai District, actively feeding on krill within 500–1000 m of each other between 60 and 180 m depth clines in May 2010, and on 2 days in June 2016 individuals were sighted feeding off Tulek Sambang and Tulek Sulaiman, including a possible mother and calf. The northernmost record comes from the Whale Stranding Indonesia online database, reporting a stranded 3 m neonate calf at Kampung Sensong, Sangihe, North Sulawesi, a location approximately 200 km south of the Philippines Archipelago (Whale Stranding Indonesia12); although recorded as B. edeni, examination of photographs indicates this is very likely B. omurai based upon its small size and dorsal fin shape.
Australia
There have been several accounts recently identified as Omura’s whales along the northwest coast of Australia from Exmouth into the Timor Sea. The only genetically verified specimen and the most western comes from a stranding of a 5.68 m juvenile female in March 2015 at Exmouth at 113.94°E (Ottewell et al., 2016). There were at least nine additional photographic accounts identified from, southwest to northeast: the Northwest Cape/Exmouth region at ca. 114.13°E (in May 2017, March 2018, Irvine, personal communication to SC; and April 2018, Ningaloo Aviation13); the Pilbara region at 117.1°E (three encounters from January to March 2010, Irvine, personal communication to SC); north of the Lacepede Islands at 122.16°E in November 2009 (Wildlife Images14); on the Heywood shoals at 124.06°E (Centre for Whale Research15) and in the Timor Sea at 127.39°E in October 2010 (Wildaires16).
There is also extensive acoustic documentation from northwestern Australia, at least from Exmouth northeast into the Timor Sea to north of Darwin, corroborating the range documented by the above records. Initially, examination of acoustic records (by SC) publically available from the Integrated Marine Observing System (IMOS17) indicated frequent detections of vocalizations on recordings off the northwest coasts of Pilbara and Kimberley between 2012 and 2013 that are very similar to those documented as Omura’s whale song in Madagascar (Cerchio et al., 2015). Given the strong similarities in acoustic structure and rhythmic repetition between these signals and Madagascar Omura’s whale song (Figure 4), we attributed these vocalizations to Omura’s whale. Review of the IMOS recordings indicated presence of these vocalizations during 7 of 11 months at Pilbara and 10 of 11 months at Kimberley region between November 2012 and September 2013. Independently, Erbe et al. (2017) also attributed these same signals to Omura’s whales based upon comparison to spectrograms and acoustic characteristics reported in Cerchio et al. (2015), stating that they are frequently recorded on Australia’s Northwest Shelf and were accompanied by photographic evidence of Omura’s whales, but report no detail on location(s). McCauley (2009) was the first to report on these vocalizations, attributing them to Bryde’s whales before the description of Omura’s whale song by Cerchio et al. (2015) and the attribution of Erbe et al. (2017); song was documented across an extensive range from at least ten recording sites in the waters between Exmouth (21.58°S 113.90°E) to north of Darwin (approximately 9.5°S 129°E). McCauley (2009, 2014) reports year-round presence of these song phrases from at least three sites, off Scott’s Reef, northwest of Broome and in Joseph Bonaparte Gulf. In an independent effort McPherson et al. (2016b) documented acoustic evidence of Omura’s whales in the north-west Timor Sea relatively close to Scott’s Reef area in 2011. McPherson et al. (2016a) report Omura’s whale vocalizations much further east in the Timor Sea in all months of the year from July 2014 to July 2015, describing movement of whales in and out of the area.
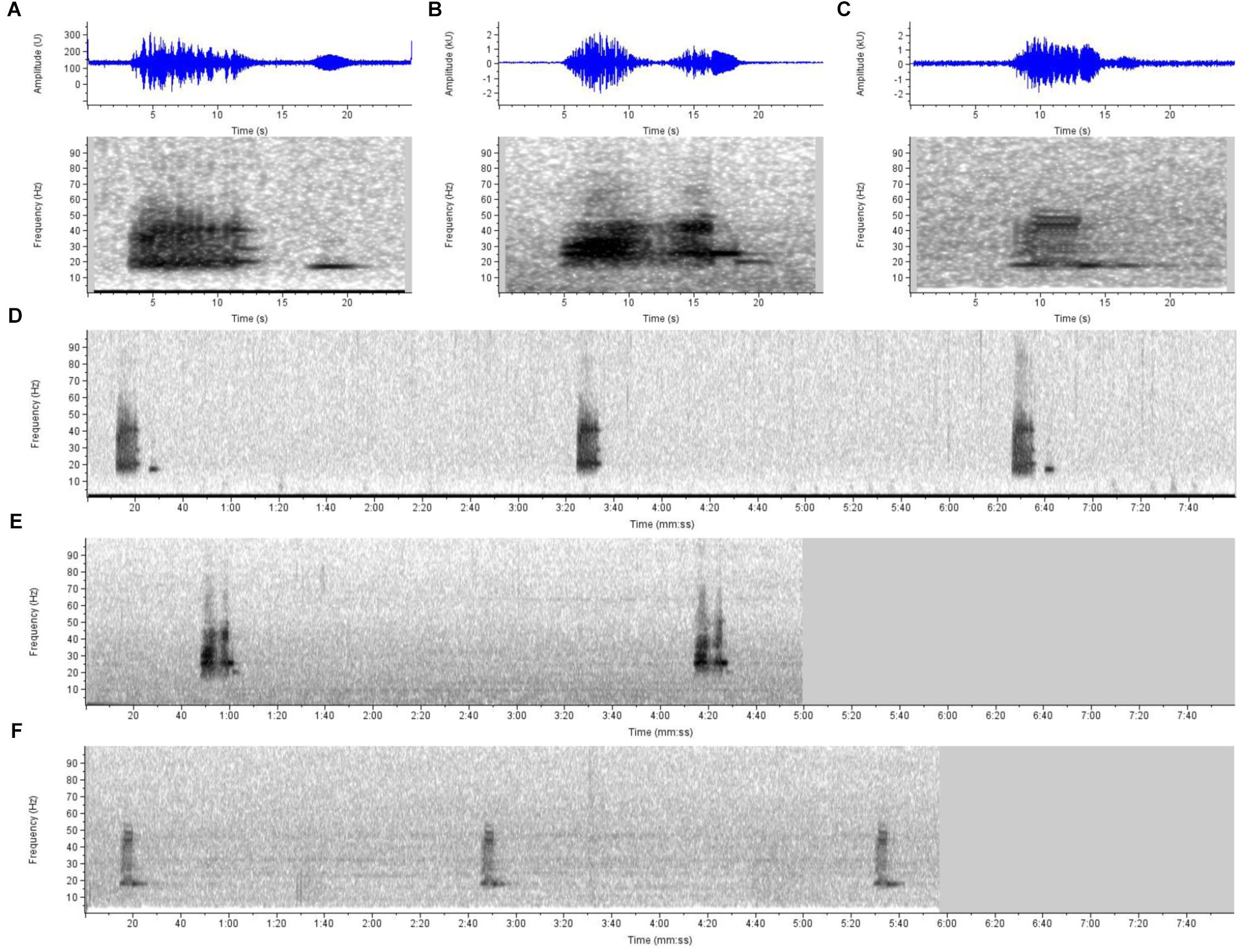
Figure 4. Sound spectrograms and waveforms illustrating similarities of song vocalizations attributed to Omura’s whales from three disparate sites in the species’ range. Shown are 25-s spectrogram and waveform (in blue) examples of (A) a single song phrase from northwest Madagascar, as described in Cerchio et al. (2015) with confirmed attribution to Omura’s whales, and similar vocalizations from (B) the northwest coast of Australia (from open IMOS acoustic data, Kimberley site), and (C) the Chagos Archipelago (near Diego Garcia) in the central Indian Ocean as described in Sousa and Harris (2015) as a baleen whale of unknown species identification [contributed by D. Harris from open Diego Garcia Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO) acoustic data]. Also shown are typical sequences of the vocalizations from (D) an 8-min sample from Madagascar, (E) a 5-min sample from Kimberley, Australia, and (F) a 6-min sample from Diego Garcia, illustrating similar repetition rates of vocalizations in each region. Due to the similarities in time-frequency structure and repetition rate of the song phrases, and distinctiveness from other known baleen whale vocalizations, we attribute the Chagos and Northwest Australia recordings to Omura’s whales based on the confirmed description from Madagascar. Recordings were converted to a standardized sample rate of 2000 Hz, and spectrograms generated at frame size of 4096 samples, 95.1% overlap and Hann window, for a frequency and time resolution of 0.5 Hz and 200 ms, respectively.
The first known account in Australia is also the most southern record for the species, a stranding of a 10.2 m individual of unknown sex in January 2000 at Black Point, Yorke Peninsula, Gulf St Vincent, South Australia, near Adelaide (Yamada et al., 2006b; Kemper, personal communication to TKY). Given the difference with all other known records and habitat, this specimen is somewhat anomalous in its extreme southern latitude (34°37.6′S) and colder water regime. Therefore we believe this is an extralimital record. Although far from the northwest Australian accounts above, it is grouped in the same regional subdivision since the Great Australian Bight is more contiguous with the East Indian Ocean than with Oceania (see below).
Oceania (see Figure 3 for Detail)
Australia
In addition to the accounts from south and northwest Australia, there are two recent photographic accounts off the northeast coast of Australia, in the Great Barrier Reef Marine Park, off Mission Beach, Queensland in November 2016, and off Port Douglas, Queensland in December 2016 (Great Barrier Reef Marine Park18, Earth Touch News Network19, Jones and Meager, personal communication; photographic evidence verified by SC). These are the first records off eastern Australia (Oceania Region), although there had been several previous accounts off northwestern Australia (covered above in the East Indian Ocean region).
Solomon Islands
Among the 459 whales taken in the 1976–1979 Bryde’s whale scientific permit catch, it was noted that seven specimens from near to the Solomon Islands were markedly smaller than from other areas (Ohsumi, 1980). These, along with the two Cocos (Keeling) Islands specimens (see above), were later found to be genetically divergent from other populations of Bryde’s whales thus gaining the label of “small” or “small form” Bryde’s whale (Wada and Numachi, 1991; Yoshida and Kato, 1999). Wada et al. (2003) eventually described these as Omura’s whales, linking them genetically to the holotype specimen from Japan. It is worth noting that the reported positions for six of these catches were in deep water of the Solomon Sea, ca. 200 km west of Isatabu (Guadalcanal). In addition, stomachs of five specimens all contained Euphausia diomedeae (Kawamura, 1980), a deep water diurnally migrating krill found at 500 m+ during the day and shallower at night, which infers that those animals were foraging in deep water. Oremus et al. (2011) reported three modern sightings of a small balaenopterid they believed to be Omura’s whales, noting a near-coastal distribution, 6–10 m size and lack of rostral ridges; these were off the west coast of Malaita Island in November 2009, two feeding animals in Sandfly Passage of the Nggela (Florida) Islands, and on the northeast side of Isatabu in November 2010. Oremus et al. (2011) present a photo of one animal in which the white right lower jaw is visible through the water, corroborating the species attribution; additional photos of four individuals on three separate days (Oremus, personal communication, verified by SC) further corroborate the species attribution based on dorsal fin shape and some discernable pigmentation. Notably, all four individuals were heavily pot-marked with cookie-cutter shark bites, a condition that is unusual in examined photographs from most other regions, and suggests a distribution that includes deep water for this population as also indicated by the Japanese catch specimens.
France/New Caledonia
Garrigue and Poupon (2013) include Omura’s whale in their cetacean field guide for New Caledonia, with a brief description (in French), photographs and illustration that pre-date the detailed documentation of Cerchio et al. (2015) off Madagascar. Between 1997 and 2014, numerous unquantified photographic records were collected off southern New Caledonia during surveys for humpback whales; however, without a genetic verification of species, the attribution of Omura’s whale was considered provisional and not published (Garrigue, personal communication to SC). It is clear from examination of photographs (by SC) that a substantive database of individual identifications may exist for this Omura’s whale population. Additional photographic documentation for the same locale comes from a sighting in May 2012 (Marine Education and Research Society20). Additionally, during aerial surveys for marine megafauna in November 2014, three records were made off the eastern coast of New Caledonia (Van Canneyt et al., 2015). It appears evident that there exists here an accessible resident population for which a long-term study has essentially commenced (as also off northwest Madagascar, see below). It is also noteworthy that all photographs examined from the above accounts show animals that are heavily scarred from cookie-cutter shark bites, indicating distribution of this population into deep water.
Western North Indian Ocean (see Figure 5 for Detail)
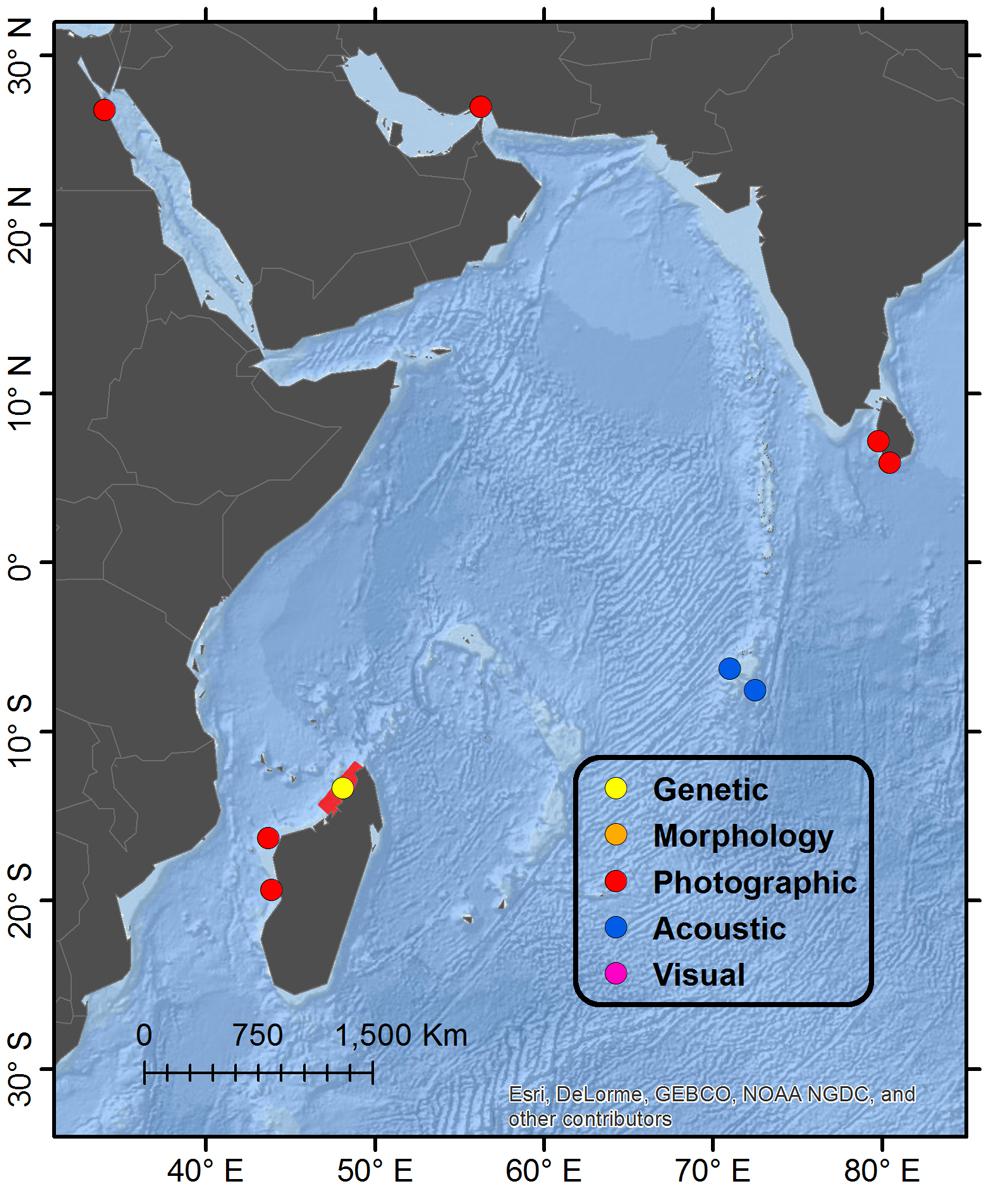
Figure 5. Detail map of western Indian Ocean regions. See Figure 1 for description of Legend entries. The range of four satellite-tagged Omura’s whales off the northwest coast of Madagascar is highlighted in red (since they were photographically identified during tagging), in addition to single positions for other accounts.
Iran
Based on direct external examination and photographs of a stranded specimen from Qeshm Island, Strait of Hormuz, Iran, Ranjbar et al. (2016) identified a 3.97 m calf rorqual as an Omura’s whale, however, no specimens were preserved from this whale. Omura’s whales may not be common in this region as none were found nearby among 18 specimens from Oman that were genetically identified as either B. edeni edeni or B. edeni brydei (Kershaw et al., 2013).
Sri Lanka
The first likely record from Sri Lanka is a 7.5 m whale taken in fishing gear off Negombo in August 1985 (for photographs see Leatherwood and Reeves, 1989, p. 104, Figures 27b,c; and Ilangakoon, 2002, p. 26). Both Leatherwood and Ilangakoon examined this specimen after it was landed on the beach and flensed. Since the Omura’s whale was described in 2003, there was no means to diagnose the species during this examination. Brownell et al. (2017) reviewed all the details of the Negombo whale and believe it was actually B. omurai due to its small size and baleen plates that are white for anterior 20–25% and then all black, which do not fit with either fin or Bryde’s whales. The first confirmed sighting of a living Omura’s whale was observed and photographed off the south coast in February 2017 (de Vos, 2017); examination of additional photographic evidence originally identified as Bryde’s whale indicated at least one other encounter with Omura’s whale in the same area in 2015 (de Vos, personal communication, confirmed by SC). These repeated sightings suggest there may be a local population off the south and west coasts of Sri Lanka.
Red Sea (see Figure 5 for Detail)
Egypt
There is one photographically documented whale from April 2009 observed near Shaab Saiman, close to Port Safaga about 53 km south of Hurghara in the Red Sea (Notarbartolo di Sciara et al., 2017, Daniel Balke21). This record in the extreme north of the Red Sea is highly intriguing given its remoteness from other records, and questions whether it is an accidental vagrant or whether there exists an unrecognized population in the Red Sea that warrants further investigation. Most other Balaenoptera sp. accounts in the Red Sea were attributed to Bryde’s whale; however, the authors state that it cannot be excluded that one or more accounts attributed to B. edeni may in fact be B. omurai (Notarbartolo di Sciara et al., 2017).
Western South Indian Ocean (see Figure 5 for Detail)
Madagascar
A study population of Omura’s whale was identified off the northwest coast of Madagascar while conducting surveys of cetacean diversity from 2011 to 2014 in the Nosy Be region (Cerchio et al., 2015). A total of 247 encounters have been recorded between 2011 and 2016, primarily during the months of October through December, and 20 individuals have been genetically sequenced; passive acoustic monitoring from December 2015 to November 2016 indicated presence of Omura’s whale song in all but 1 week of the year; satellite tagging of four whales in November/December 2016 indicated a restricted range during a period of 2 months along an approximately 400km stretch of coastline from 12.06°S to 14.81°S latitude (highlighted in Figure 5), centered on the tagging site of Nosy Be (13.40°S) (Cerchio et al., 2018). These data along with individual photographic recaptures across years suggests a resident local population that may be relatively small. Additional photographic records from Madagascar off Chesterfield Island and Ambozaka in July 2016 indicate a southerly distribution at least to 16.33°S (Filmater, personal communication, verified by SC), however, the relationship of these animals to the Nosy Be population is not known. Extensive long-term surveys in the southwest Anakao and northeast Antongil Bay regions indicated no reports of Omura’s whale or Bryde’s-like animals (Cerchio, personal observation), so the distribution around Madagascar is possibly limited to the central west and northwest coasts.
Chagos Archipelago – British Indian Ocean Territory
Sousa and Harris (2015) report on two vocalizations of unknown origin off Diego Garcia in the Chagos Archipelago (north and south locations at 6.3°S and 7.6°S) attributing them to a baleen whale and speculating they may represent undescribed blue whale vocalizations. One of these, the “Diego Garcia Croak” (DGC), is very similar to Omura’s whale song documented off northwest Madagascar (Cerchio et al., 2015), allowing for differences due to signal-to-noise ratio of the available recordings (Figure 4). The song phrase consists of two units, the first being amplitude modulated and broadband from 15 to 50 Hz, and the second being tonal at approximately 18 Hz; the main demonstrable difference between the Madagascar song phrase and the DGC is the absence of a four sec gap between the two units that is a stereotyped feature of the Madagascar song, along with overall duration (Figure 4). The repetition rate of the song phrase is also very similar being 186 s (s.e. 18 s) from Diego Garcia (Sousa and Harris, 2015), and 189.7 s (s.d. 19.04 s) from Madagascar (Cerchio et al., 2018; from 118 individual series ranging from 20 to 250 repeated phrases). It is nearly certain that the DGC vocalization is produced by a population of Omura’s whales that may reside year-round at Diego Garcia/Chagos Bank with peaks of occurrence in both the Austral winter and summer, according to the acoustic monitoring presented in Sousa and Harris (2015). Although the Chagos detections were made in deep water pelagic habitat (>1000 m), the hydrophones were within 20 km of extensive shallow water bank and island habitat (<100 m), so do not necessarily represent pelagic accounts.
Eastern North Atlantic Ocean (see Figure 6 for Detail)
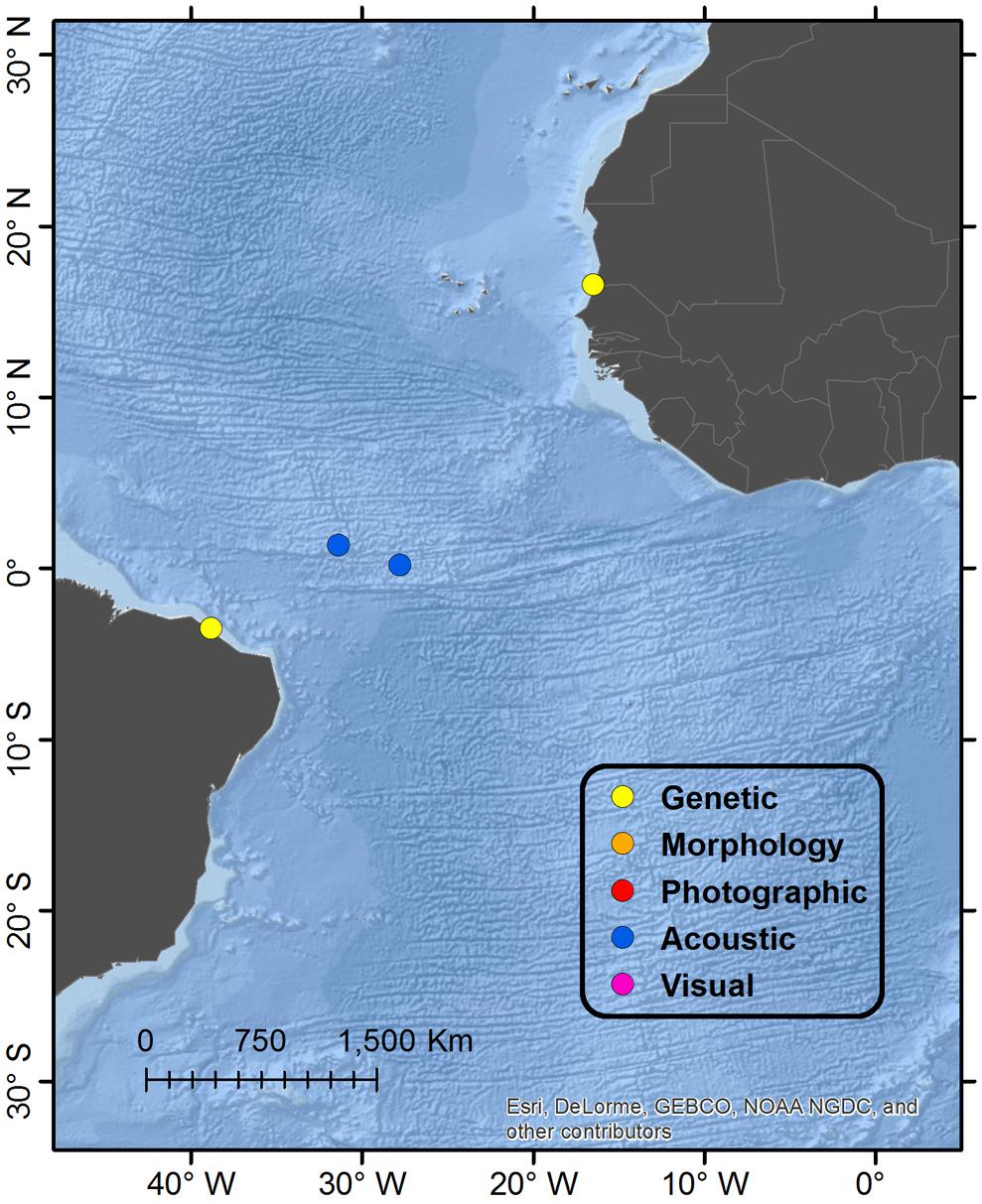
Figure 6. Detail map of Atlantic Ocean regions. See Figure 1 for description of Legend entries.
Mauritania
A small baleen whale with a total length of 3.98 m stranded in Diawling National Park and was identified genetically as an Omura’s whale (Jung et al., 2015). This currently represents the only verified account from the North Atlantic Ocean basin, and to date there exist no documentation in the eastern South Atlantic Ocean basin. Given that this specimen was a calf and likely still dependent on its mother prior to stranding, the authors argue that the specimen more likely indicates the existence of a local population off Mauritania as opposed to a vagrant extralimital occurrence. With the current known distribution of the species, this would infer a strongly discontinuous range with a large gap in distribution between the southwest Indian Ocean (Madagascar) and the northeast Atlantic Ocean; however, given the paucity of data and survey effort off much of the coasts of Africa, there is clearly potential for existence of other populations in both west and east Africa.
Western South Atlantic Ocean (see Figure 6 for Detail)
Brazil
A single stranded whale was genetically identified as an Omura’s whale from Pecém beach, São Gonçalo do Amarante, Ceara State in northeastern Brazil. The specimen was incomplete (missing most of the skull) but estimated “within a few centimeters” to be a 4.16 m female (Cypriano-Souza et al., 2016), and therefore a calf still dependent on its mother. Together with the Mauritania account, also of a dependent calf, the authors argue that these strandings more likely represented a regional population or populations as opposed to vagrants from another ocean basin, furthering the evidence for extension of the species range into the Atlantic Ocean. There is recent acoustic evidence of Omura’s whale near the São Pedro and São Paulo Archipelago on the mid-Atlantic Ridge approximately 900–1000 km northeast from the Brazilian coast (Moreira et al., 2018); Omura’s whale song was recorded on two hydrophones, anchored in 3000+m depth and monitoring the deep-sound channel at 780 m, and located 190 km east-southeast and 230 km west-northwest from the Archipelago (the nearest shallow water) during February to December 2013. Although it is possible that these signals traveled a great distance in the deep-sound channel, it is likely that the vocalizing whales were in deep water pelagic habitat.
Discussion
Our understanding of the biology of Omura’s whale has progressed substantially since the publication of Wada et al. (2003) describing the species. During the decade after this first description, steady work (primarily by the original research group, TKY and colleagues) expanded the knowledge of the species range to establish its presence throughout the western Pacific and eastern periphery of the Indian Ocean. With the discovery of a tractable study population in Madagascar and publication of a detailed physical description and high quality field photos (Cerchio et al., 2015), the rate of new reports increased precipitously between 2015 and 2018 (Figure 7). Furthermore, during review of photographic accounts reported here, it was noted that the diagnostic pigmentation patterns described for the Madagascar population are present in all regions where photos or videos were examined and verified (including Madagascar, Red Sea, Sri Lanka, Andaman Sea, Thailand, Indonesia from West Sumatra to Raja Ampat, northwest Australia, Philippines, Taiwan, northeast Australia, Solomon Islands and New Caledonia). Throughout this broad range of photo-documented accounts spanning 123° of longitude and 49° of latitude, the common diagnostic features present included the asymmetric pigmentation of the jaw, lightly pigmented right side blaze with four bisecting dark stripes, asymmetric chevron on both sides, lightly pigmented leading edge of pectoral fin, delicate falcate dorsal fin and single prominent rostral ridge; variation in these characteristics among sites appeared no greater than observed between individuals as presented in Cerchio et al. (2015, see Figure 3, electronic Supplementary Material Figures S1–S3 of that publication), and observed in over 200 encounters off Madagascar (Cerchio et al., 2018). Thus we conclude that these characteristics are universal to the species, and strong indicators to distinguish from other similarly sized Balaenoptera species (dwarf minke, Antarctic minke and Bryde’s whales).
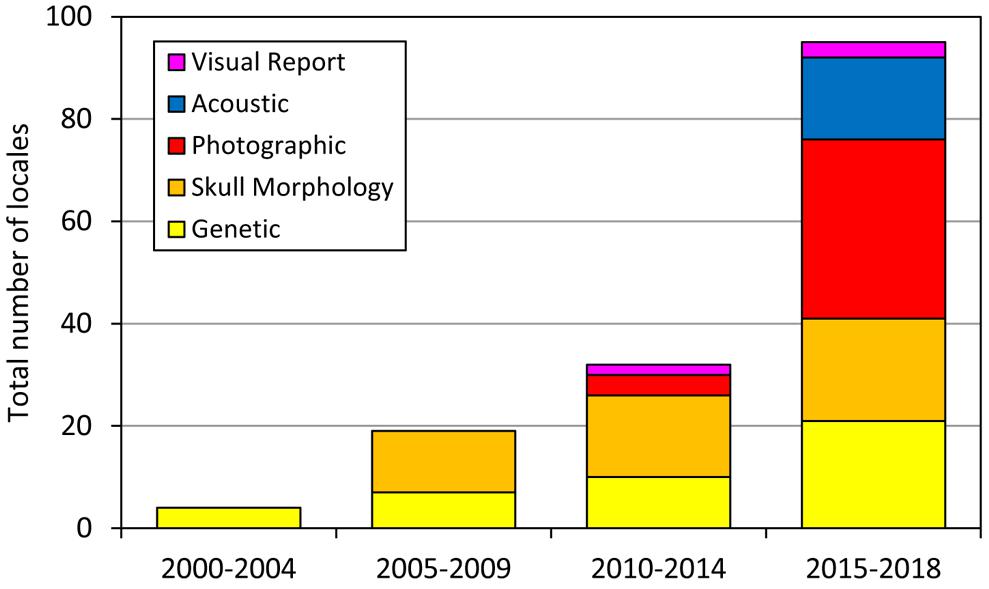
Figure 7. Cumulative number of locations with accounts of Omura’s whales since the first description by Wada et al. (2003), illustrating an increase in reports, particularly photographic and acoustic, after publication of detailed descriptions of physical appearance and vocalizations in Cerchio et al. (2015). Accounts are tallied by location, so that reports of multiple accounts from a given locale are counted as a single addition to the sum.
The sum total of encounters reported here, combining photographically identified individuals at sea, strandings that have been genetically or morphologically identified as Omura’s whales, and acoustic documentation of Omura’s whale song, has resulted in a marked expansion of the species’ range well beyond the originally suspected Indo-Pacific core region. Latitudinally, all records occur between 35°N and 35°S, with 83.2% falling within the tropics between 23.5°N and 23.5°S. The Northern Hemisphere warm temperate records are primarily west North Pacific accounts (n = 24 of 26) in Japan, Korea and the East China Sea, a region strongly influenced by the warm Kuroshio Current from the south. Conversely, the only Southern Hemisphere record that is south of the Tropic of Capricorn is a stranding near Adelaide, Australia, that is almost certainly extralimital. Currently it is known from all ocean basins with the exception of the central and eastern Pacific. The majority of accounts remain in the eastern Indo-Pacific region, and examination of current accounts reported here suggest a continuous distribution from southeast Asia through the shallow seas of the Indochinese Peninsula, Indonesia and the Philippines, to the Timor Sea and northern Australia, and at least to the Solomon Sea and New Caledonia. Absence of accounts in some parts of this range (e.g., off Papua New Guinea) may be solely due to lack of effort or confusion with Bryde’s whales. Lack of accounts further east than New Caledonia (e.g., Fiji, Samoa, Cook Islands, and French Polynesia) may similarly be due to lack of effort or confusion with Bryde’s whales, or may represent eastern limits of the species range. That there are no records from the eastern Pacific, despite extensive cetacean survey effort in some regions (e.g., the Eastern Tropical Pacific and coasts of Mexico, Central America, and northern South America; Hamilton et al., 2009; Forney et al., 2012) suggests there may indeed be an eastern range limit in the Pacific. It is worth noting that other predominantly coastal species have a similar barrier to dispersal across the Pacific (e.g., the genera Sousa, Orcaella, and Neophocaena). Conversely, moving west from the Indo-Pacific region, reports in the western Indian Ocean are steadily increasing. Currently the range appears fragmented along the rim of the Indian Ocean from the Andaman Sea, to Sri Lanka, Persian Gulf/Gulf of Oman, and to Madagascar, along with mid-Indian Ocean occurrence in the Chagos Archipelago. Lack of reports from vast areas along the Bay of Bengal, Indian subcontinent, Arabian Sea and the eastern coast of Africa may also be due to lack of effort; however, 59 samples of “Bryde’s whales” from Bangladesh (n = 30), the Maldives (n = 8) and Oman (n = 18) genetically assessed by Kershaw et al. (2013) were all classified as either B. edeni edeni or B. edeni brydei. Therefore the distribution in this part of the range may indeed be more fragmented than it appears to be in the eastern Indo-Pacific region. Its presence in both the North (Mauritania) and South (Brazil) Atlantic has been documented, however, these reports remain sparse, with currently no accounts in the southeastern Atlantic along the west coast of Africa; it is as yet unclear whether this is a consequence of less effort to identify the species in this part of its range, or whether it is actually less abundant in the Atlantic Ocean. It is possible that in the coming years further discoveries will indicate a tropical/warm-temperate worldwide distribution.
The current described range suggests a center of distribution in the eastern Indo-Pacific and potentially a recent range expansion out from this region to both the east and west in tropical/warm-temperate waters. A recent expansion hypothesis would be congruent with the observation of low genetic diversity observed in mitochondrial DNA among several disparate regions from which genetic material has been obtained. A review of published literature for which a comparable approximately 400 base-pair sequence of mtDNA control region was analyzed revealed only four haplotypes among eight locations spanning the known range [Japan, Solomon Sea, Cocos (Keeling) Islands, Wada et al., 2003; Korea, Kim et al., 2018; East China Sea, Yang et al., 2002; Madagascar, Cerchio et al., 2015; Mauritania, Jung et al., 2015; Brazil, Cypriano-Souza et al., 2016]. This represents the results from only a single genetic marker, so conclusions about global genetic diversity can only be considered preliminary, but the mtDNA control region is documented to have considerable variation in other species. This apparent lack of range-wide genetic diversity is somewhat at odds with the established ancient origin of the lineage, with divergence from the sei whale/Bryde’s whale clade estimated between 7 and 17 million years ago (Sasaki et al., 2006; Marx and Fordyce, 2015; Slater et al., 2017). Low population genetic diversity could be explained by a relatively recent severe bottleneck, range contraction and prolonged period of small population size, followed by subsequent pan-tropical range expansion. Further work sampling populations broadly, comparing genetic diversity at multiple markers across the range, and assessing models of paleo-climate change should be conducted to assess this hypothesis.
There is a strong tendency toward a coastal and neritic water distribution, although there are several pelagic water records as well. The majority of accounts are on continental shelves and shallow seas throughout the documented range. Field research in Madagascar indicates a strong preference for shallow water, on-shelf habitat, with satellite telemetry and acoustic monitoring data indicating only infrequent short ventures of individuals off the shelf into adjacent deep water (Cerchio et al., 2015, 2018). Globally, the predominance of shallow water records may in part be due to a detection bias, as deep water records and accounts around small oceanic islands are noted from the original whaling specimens off the Cocos (Keeling) Islands and in the Solomon Sea, as well as recent acoustic documentation off Brazil on the mid-Atlantic Ridge. Also individuals from several populations have been noted to bear heavy scarring from cookie-cutter sharks, including from the Solomon Islands, New Caledonia, West Sumatra, East Kalimantan and possibly the Philippines; since cookie-cutter sharks of the genus Isistius are deep water animals, this indicates at least some use or crossing of deep water pelagic habitat. This appears to be population specific, however, since extensive photographic documentation off Madagascar indicates almost a complete absence of cookie-cutter shark scars, whereas other deep water species in the region (e.g., pantropical spotted dolphins, Stenella attenuata) frequently have such scars (Cerchio, unpublished data). It was also noted in our review of photographic accounts that individuals from many regions are similarly free of cookie-cutter shark scars (e.g., Sri Lanka, Andaman and Nicobar Islands, Timor Sea). Therefore, it appears from this review that the degree to which Omura’s whales are restricted to shallow waters varies across populations, with some that may predominantly utilize shallow habitat whereas others only partially so. It is likely that this is driven by available prey resources, and that diet and feeding habits will vary between populations with different habitat tendencies. A predominantly near coastal distribution places Omura’s whale at risk of impact from anthropogenic activities throughout its range, and its tropical distribution in often remote and poorly studied and monitored areas makes adequately documenting and assessing threats challenging.
Global Threats
Thomas et al. (2015) provided an overall review of the threats to baleen whales. They specifically identified the following as the main threats: entanglement/entrapment, ship strike, whaling, pollution, disease, habitat degradation from oil spills, the cumulative impacts of anthropogenic noise and other stressors, and the short- and long-term effects of climate change and ocean acidification on marine ecosystems. However, bycatch (entanglement or entrapment in fishing gear) and ship strikes are the primary threats at the population level and they concluded that those threats were most significant for populations already at critically low numbers.
Given the primarily coastal and shallow water distribution of Omura’s whale, the species is in particular risk of anthropogenic interactions and threats relative to more oceanic balaenopterids. Thus we expect that Omura’s whale populations have a relatively high probability of impact from entanglement and bycatch in local fisheries, ship strikes, coastal development and coastal industry. Furthermore, current evidence indicates that populations are non-migratory with local, potentially restricted ranges in low latitude coastal waters, for example as indicated by long-term acoustic monitoring off Madagascar (Cerchio et al., 2018), the Chagos Archipelago (Sousa and Harris, 2015) and northwestern Australia (McCauley, 2009, 2014). Resident populations of cetaceans face different conservation challenges compared to migratory baleen whales; for instance, seasonal restrictions on potential threats (i.e., seismic exploration, shipping lanes, or bycatch-prone fishing gear) are not effective on a resident population that is present year-round. Moreover, the exclusively tropical and warm-temperate distribution results in many documented populations occurring off the coasts of developing countries, for which there is typically little regulation of such threats and anthropogenic activities. If results from initial population studies in Madagascar bear out to apply range-wide, and the species is characterized by small, localized populations, with low range-wide genetic diversity (Wada et al., 2003; Cerchio et al., 2015; Cypriano-Souza et al., 2016), then they may be particularly vulnerable to such anthropogenic impacts that could cause small populations to decline. The distribution of the species in typically remote and poorly surveyed regions exacerbates the problem, in that both occurrence of interactions and magnitude of impacts is difficult to impossible to document.
The following are examples of currently documented threats faced by the various populations of Omura’s whales reported here:
Ship Strikes
There are two known records of ship strike mortalities involving Omura’s whales. The holotype specimen from southwestern Japan was a ship strike (Oishi et al., 2004), and a specimen from Manila Bay, Philippines was carried into the port on the bow of a ship (Dolar, personal communication). Although there are no other confirmed cases of ship strikes, when Omura’s whale populations occur in close proximity to human population, shipping lanes and industrial centers (such as off southern Japan, coasts of China, Taiwan and Hong Kong, East China Sea, South China Sea, Malacca Strait, Sri Lanka, and Persian Gulf), then we expect a high risk of ship strike due the typically shallow coastal habitat preference of many populations and potential overlap with ship traffic.
Incidental Fisheries Bycatch/Entanglement
Bycatch of Omura’s whales in local fisheries has been documented in several regions. Three of the six specimens known from Japan were entanglement mortalities; the two records from Korea are indicated as “bycaught in fishing gear” in nearshore waters (Kim et al., 2018); an immature male of 4.4 m length was bycaught in small-mesh herring seine nets in the Gulf of Thailand, Songkhla Province (Adulyanukosol et al., 2012); the first specimen of Sri Lanka from 1985 was taken as bycatch in coastal waters and de Vos (2017) reported a scar that is suggestive of entanglement on the rostrum of a live animal off southern Sri Lanka. The relatively high number of bycatch (and ship strike) mortalities reported from Japan and Korea is likely due to an increased likelihood of detecting such events in a highly populated and developed region. For much of the range, such as in the southwest Indian Ocean and the extensive coasts of Indonesia, the remoteness and/or lack of development makes detection and monitoring of bycatch unlikely. Therefore we expect that bycatch and entanglement across the range is likely grossly under-reported.
Directed Hunting
Indigenous local hunting on Omura’s whales has been documented in at least two regions with indications of potential population level impacts. In the Indonesia range, hunting of medium-sized balaenopterids was first discussed by Weber (1923), relatively widespread from the north coast of Java, to the Lesser Sundas, from Komodo east to Lamakera, Solor; photographs of skulls confirmed that at least some of the catch were Omura’s whales. Additional evidence of indigenous hunting on Omura’s whales in Indonesia comes from a photograph of a landed whale from Lamakera village on Solor Island taken between 1915 and 194422 (Egami and Kojima, 2017) that strongly resembles a young Omura’s whale in size and shape. Mustika (2006) assesses subsistence whale hunting in the Savu Sea, bounded by Flores, Sumba and Timor Islands, and identified the villages of Lamalera on Lembata Island, and Lamakera on Solor Island as having hunted whales at least back to the early 17th century. Sperm whales are the main target of Lamalera, where the hunt is still important to the community, but a reduction in catch of whales has forced a switch to smaller cetaceans, whale sharks and manta rays; this includes documentation of an unidentified baleen whale (rorqual) in 2005. Lamakera hunters preferentially targeted small rorquals (<10 m), but Mustika (2006) could not identify to species, nor were Omura’s whale well described at the time of the research; given the size description along with other accounts in the region it is likely that the hunt was at least partially if not predominantly on Omura’s whales. Moreover, historical photographs, presented by Weber (1923) of a skull, and Egami and Kojima (2017) of a landed whale, indicate that Omura’s whales were hunted in Lamakera. Mustika (2006) reported that whale hunting appeared to be no longer important to villagers on Lamakera, with less than three whales caught each year, and only one or two in 2004. Mustika (2006) concluded that the Lamalera hunt may still be a threat to local whale populations, and the impact of the Lamakera hunt is unknown; there was at least some anecdotal evidence of reduced occurrence of whales in the passages around the islands associated with both villages. Therefore it seems likely that the Omura’s whale population in the Savu Sea region has been at least historically impacted and may continue to be at risk from this hunt.
A traditional shore-based whaling operation in the Bohol Sea, Philippines, dated back to the late 19th Century into at least the 1990s at Pamilacan Island, Lila on Bohol Island, Sagay on Camiguin Island, and Salay on Misamis Oriental (Acebes, 2014). There was also a period of commercial whaling from ca. 1981 to at least 1987 that was conducted by Japan out of the Bohol region but hunted in distant waters (Acebes, 2014). Both of these operations took what was considered at the time to be Bryde’s whales and pygmy Bryde’s whales, but it is possible if not likely that the traditional catch within the Bohol Sea were predominantly Omura’s whales (Dolar et al., 1994; Perrin et al., 1996; Yamada et al., 2008; Acebes, 2013). Dolar et al. (1994) reported over 100 whales taken between 1985 and 1993 from Pamilacan alone, and that whaling stopped in Lila by 1986 due to disappearance of whales. Acebes (2009, 2013) found that inhabitants of all the Bohol Sea villages reported a decline in the abundance of the local whales (along with other marine megafauna) from historic numbers, and it is possible that this is in part due to the local hunting pressure; however, the author emphasizes that the exact cause of the decline cannot be determined and may be due to cumulative impacts from other stressors on the populations (Acebes, 2009, 2013). In reference to Bryde’s whales, Acebes (2009, 2013) discussed migratory patterns from Japan and the probable impact of activities in other parts of the range, and concludes that the small number of takes in the Bohol Sea were unlikely to impact the population. This assessment of impact of hunting pre-dates information from Madagascar indicating that Omura’s whales are non-migratory, locally resident, and likely have small population sizes (Cerchio et al., 2015, 2018). Although the specific habits of the Omura’s whale population in the Bohol Sea region remain unknown, if the characteristics of the Madagascar population prove to be common throughout the species range then it is conceivable that the Philippine hunts could have had a serious impact on the population. Therefore, the decline reported by Dolar et al. (1994) and Acebes (2009, 2013) may be indicative of a severe impact on the local population, and emphasize the threat posed by even moderate levels of hunting on a small, resident population of non-migratory baleen whales.
In addition to these local hunts, it is possible that Omura’s whales may have been taken in western Pacific hunts off coastal western Japan. Omura (1962, 1977) reported that Bryde’s whales taken between 1955 and 1960 off the west coast of Kyushu (southernmost main island of Japan) were smaller than takes from three other regions and suggested they belong to another group or population. It is possible that at least some of these could have been Omura’s whale, with reported sizes ranging from 10.3 to 13.3 m. However, it should be noted that Yoshida and Kato (1999) reported that 14 specimens taken from the same general area in 1974 (during Japanese land-based whaling operations off Goto Islands) grouped into a clade that would identify as B. edeni edeni in mtDNA phylogenies. Therefore, it is clear that Bryde’s whales ranged in this area as well and if Omura’s whales were taken it was likely a mix of the two species. In addition Kondo (2001) reports that coastal whaling catcher boats took Bryde’s whales 40 miles off Shionomisaki, Wakayama Prefecture during June 1976. These whales were small and two females (7.9 and 8.5 m) were sexually mature as indicated by corpora albicantia in the ovaries, and thus we believe these were likely Omura’s whales. Therefore, this population has probably been subjected to some hunting pressure, but further assessment needs to be done to understand the impact and current status.
Petroleum Exploration and Production (E&P)
Seismic exploration overlaps extensively with known ranges of Omura’s whale populations at least off the west and northwest coast of Australia, and the northwest coast of Madagascar. In Australia, McCauley (2009) documents simultaneous detection of Omura’s whale song and seismic airgun pulses at Scott’s Reef off northwest Australia, at times with complete masking of the Omura’s whale frequency bandwidth. Similarly, McPherson et al. (2016a) documented seismic survey pulses overlapping Omura’s whale song nearly 1000 km away in the eastern Timor Sea, suggesting that this population has been exposed to surveys potentially at close range in shallow water over an extensive portion of its range. The same is likely true of the Madagascar population in conjunction with recent seismic exploration activities in the Majunga, Ampasindava and Ambilobe lease blocks which overlap with shallow water habitat (Cerchio et al., 2015), although it has not been monitored or assessed for potential impacts. Most recently in November 2017, a major international petroleum company (British Petroleum) obtained rights to four lease blocks covering 45,000 km2 in the northwest of Madagascar and plans exploration (African Energy News23, L’Express de Madagascar24). This large area of coastal and deep waters from Cap Saint-André to the Ampasindava Peninsula overlaps entirely with the range described by Cerchio et al. (2015, 2018) for Omura’s whales, and thus poses a threat to the Madagascar population if not properly monitored and regulated.
We cannot here assess the global extent of overlap between Omura’s whale populations and seismic exploration or oil production, but it is likely to be far more widespread then represented by these two highlighted regions. There is potential for disturbance of breeding and resting behavior, masking of communication, and even physiological damage if in close proximity or with long exposures of cetaceans to loud anthropogenic noises. Numerous studies on a variety of cetacean species globally have documented behavioral responses, such as changes in travel routes and distribution, and shifts in vocal display behavior to seismic airguns, echo sounders, navy sonar and ship noise (Nowacek et al., 2007; Castellote et al., 2012; Cerchio et al., 2014; Cholewiak et al., 2018). It has also been shown that noise from petroleum E&P activities and vessel traffic contributes significantly to ambient noise within the communication bandwidth of baleen whales, likely acting to mask communication and breeding displays (Clark et al., 2009; Cholewiak et al., 2018). We conclude that Omura’s whales will be particularly vulnerable to such exposure within their habitat given restricted ranges and potential lack of alternative habitat in some parts of their range. Furthermore, the shallow water nature of many populations could exacerbate the impact due to the propagation characteristics of low-frequency noise sources in shallow water (i.e., ducting).
Coastal Industrial Development
In Madagascar there is currently a mining project underway for extraction of rare earth elements (REE) from ion-adsorptive clay deposits on the Ampasindava Peninsula, adjacent to the primary habitat identified for the Omura’s whale population (Tantalus Rare Earths AG25). REE extraction from ion-adsorptive clays, using surface mining and heap leaching or in-situ leaching methods, is among the most environmentally damaging types of extraction due to destruction of habitat, groundwater contamination and mining effluent (Palmer et al., 2010; Yang et al., 2013; Lei et al., 2017). Therefore, the Madagascar project could pose grave long-term concern to the local marine environment, as well as terrestrial habitat and local communities, particularly if not well regulated (Wilmet et al., 2017, National Geographic26, Mangabay27). The resultant pollutant risk has the potential to affect the ecosystem at low trophic levels, possibly disrupting ecological processes that drive the occurrence and distribution of a diversity of marine megafauna. These effects are particularly of concern in poorly regulated operations without adequate mitigation of environmental impacts (Yang et al., 2013; Vahidi et al., 2016). The fact that the Ampasindava Peninsula was designated a New Protected Area in 2014, and the coasts adjacent to the mining project are encompassed by one of the largest Marine Protected Areas in Madagascar (the Ankivonjy MPA), underscores the challenges of protecting habitat along the coasts of developing nations, where regulation and mitigation of industrial impacts are typically poor or lacking. Much of the Omura’s whale range throughout the world is found in similar regions, therefore it is possible that coastal industrial development could pose a widespread threat to populations globally.
Conclusion
The IUCN Red List status of Omura’s whale is currently Data Deficient, and it is likely to remain so until concerted effort is made to assess status and threats to many of the probably small isolated populations throughout its range. Documented mortalities include ship strikes, fisheries bycatch, and local traditional hunting, and there exist clear threats from coastal development and industry. Given the potential for the species to be characterized by small localized populations, and the currently documented low genetic diversity throughout its range (Wada et al., 2003; Cerchio et al., 2015; Cypriano-Souza et al., 2016), it may be particularly vulnerable to impacts from existing anthropogenic threats. Our review of accounts provides a first global assessment of range that could be useful in assessing risks, by modeling habitat suitability on a global scale and mapping the overlap of Omura’s whale habitat with anthropogenic threats such as shipping traffic, fisheries and industrial activities. It is strongly recommended that focused work be conducted to locate and study local populations, assess isolation versus connectivity of nearby populations, and determine conservation status and anthropogenic threats. Currently there is only one population we are aware of that is targeted for detailed study, off northwest Madagascar (Cerchio et al., 2015, 2018). Through our accounts globally we have identified several other regions where populations appear to range in areas that might be tractable for similar study (based upon repeated live sightings and multiple reports, and the presence of some research/observational effort): eastern Taiwan; Sri Lanka; the Andaman and Nicobar Islands; the Gulf of Thailand; central Indonesia off Bali and the Komodo National Park; eastern Indonesia off Raja Ampat; the Philippines in the Bohol Sea; northwest Australia and the Timor Sea; the Solomon Islands; New Caledonia. We hope that this review will inspire more work on this enigmatic species throughout its range to address the multitude of questions that remain about its behavior, vulnerabilities and conservation status.
Author Contributions
SC conducted the research on accounts and wrote the manuscript. TKY conducted the research on accounts and assisted in writing the manuscript. RLB conducted the research on accounts and assisted in writing the manuscript. All authors gave authorization for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many individuals provided input, opinions, reports, descriptions of unpublished accounts and/or photographs or recordings of Omura’s whales from around the world, including: Jo Marie Acebes, Robert Baldwin, Richard Brown, Peter Corkeron, Danielle Cholewiak, Asha de Vos, Louella Dolar, Tomoko Egami, Nick Filmater, Claire Garrigue, Danielle Harris, Lyn Irvine, Hajime Ishikawa, Chris Jones, Catherine Kemper, Danielle Kreb, Sophie Laran, Alex Lindbloom, Loka Kawasan Konservasi Perairan Nasional Pekanbaru, Akshay Malavi, Mahi Mankeshwar, Robert McCauley, Craig McPherson, Justin Meager, Gianna Minton, Sergio Moreira, Simon Mustoe, Giuseppe Notarbartolo di Sciara, Masayuki Oishi, Marc Oremus, Bill Perrin, Bob Pitman, Renata Sousa Lima, David Stemmer, Dipani Sutaria, and Olivier Van Canneyt. Acoustic data from Diego Garcia contributed by Danielle Harris were collected as part of the Comprehensive Nuclear-Test-Ban Treaty Organization (CTBTO) International Monitoring System. Acoustic data from Pilbara and Kimberley, Australia, were sourced from the Integrated Marine Observing System (IMOS); IMOS is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy and the Super Science Initiative.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00067/full#supplementary-material
Footnotes
- ^https://www.facebook.com/media/set/?set=oa.993823800737832&type=3. This is a compilation of several posts of accounts; individual links to each separate account is available in the Supplementary Material table of accounts.
- ^https://www.youtube.com/watch?v=3JSrl2bd0-g
- ^http://www.marinemammals.in/index.php/database/sightings-strandings/details/1/4887
- ^http://www.marinemammals.in/index.php/database/sightings-strandings/details/1/5109
- ^http://www.nbcnews.com/id/27196362/ns/world_news-world_environment/t/whale-autopsy-trash-clogged-its-intestine/#.WDrrnfkrI2w
- ^https://www.facebook.com/DugongDiveCenterPH/photos/a.796355213734087/956178647751742/?type=3&theater
- ^http://www.whalestrandingindonesia.com/stranding-database.html#home/viewmaindatumdetails/56b1890ea7eb00b835ad704e
- ^http://lkkpnpekanbaru.kkp.go.id/tim-monitoring-cetacean-temukan-kemunculan-2-spesies-yang-melengkapi-6-spesies-cetacean-di-kawasan-konservasi-twp-pieh
- ^https://www.facebook.com/WhaleStrandingIndonesia/photos/944684412309516 & https://www.instagram.com/p/BF5VkQ9J9z8
- ^https://www.youtube.com/watch?v=ua1mXf-JFvs
- ^https://www.facebook.com/zoopod/videos/1290667037637149 & https://www.facebook.com/zoopod/videos/1290634154307104
- ^http://www.whalestrandingindonesia.com/stranding-database.html#home/viewmaindatumdetails/5a84d99b6f194c3d98921c40
- ^https://www.facebook.com/permalink.php?story_fbid=1664475753622059&id=958722754197366
- ^http://www.pbase.com/wildlifeimages/omuras_whale
- ^http://www.cwr.org.au/fremantle-to-hobart-blog-chapter-6.html
- ^http://au.whales.wildiaries.com/trips/10341
- ^https://acoustic.aodn.org.au/acoustic/
- ^https://www.facebook.com/GreatBarrierReefMarinePark/videos/1283672321693302
- ^http://www.earthtouchnews.com/oceans/whales-and-dolphins/worlds-most-elusive-whale-filmed-on-the-great-barrier-reef
- ^https://mersociety.wordpress.com/2012/11/07/extraordinarily-rare-whale-sighting-omuras-whale-balaenoptera-omurai
- ^https://www.youtube.com/watch?v=Y68R-7VsUvU
- ^https://commons.wikimedia.org/wiki/File:COLLECTIE_TROPENMUSEUM_Bewoners_van_kampong_Lamakera_bekijken_een_gevangen_walvis_Noordoost-Solor_TMnr_10006615.jpg
- ^https://www.africa-energy.com/article/bp-takes-four-madagascar-blocks
- ^https://www.lexpressmada.com/20/02/2018/bloc-petrolier-le-dossier-british-petroleum-boucle/
- ^http://www.tre-ag.com/operations
- ^https://blog.nationalgeographic.org/2017/08/23/proposed-rare-earths-mine-threatens-protected-forest-lemurs-and-farmers-in-madagascar/
- ^https://news.mongabay.com/2017/11/another-blow-to-troubled-madagascar-rare-earth-mine/
References
Acebes, J. M. V. (2009). Historical whaling in the Philippines: origins of ‘indigenous subsistence whaling’, mapping whaling grounds and comparison with current known distribution: a HMAP Asia Project Paper. Working Paper No. 161. Asia Research Centre, Murdoch University, Perth, 37.
Acebes, J. M. V. (2013). Hunting “big fish”: A Marine Environmental History of a Contested Fishery in the Bohol Sea. Doctoral dissertation, Murdoch University, Perth, 395.
Acebes, J. M. V. (2014). “A history of whaling in the Philippines: a glimpse of the past and current distribution of whales,” in Historical Perspectives of Fisheries Exploitation in the Indo-Pacific, eds J. Christensen and M. Tull (Dordrecht: Springer), 83–105.
Adulyanukosol, K., Thongsukdee, S., Passada, S., Prempree, T., and Wannarangsee, T. (2012). Bryde’s Whales in Thailand. Bangkok: Aksornthai Printing Co.
Aragones, L. V., Roque, M. A. A., Flores, M. B., Encomienda, R. P., Laule, G. E., Espinos, B. G., et al. (2010). The Philippine marine mammal strandings from 1998 to 2009: animals in the Philippines in peril? Aquat. Mamm. 36, 219–233. doi: 10.1578/AM.36.3.2010.219
Boden, B. P., Johnson, M. W., and Brinton, E. (1955). Euphausiacea (Crustacea) of the North Pacific. Bull. Scripps Inst. Oceanogr. 6, 287–400.
Brinton, E. (1975). Euphausiids of Southeast Asian Waters. Naga Report. Scientific results of marine investigations of the South China Sea and Gulf of Thailand, 1959–1961. San Diego, CA: University of California.
Brownell, R. L. Jr., de Vos, A., and Ilangakoon, A. D. (2017). Large whale strandings from Sri Lanka between 1889 and 2014. Paper SC/67A/HIM/11 Presented to the International Whaling Commission Scientific Committee, Histon and Impington, 13.
Castellote, M., Clark, C. W., and Lammers, M. O. (2012). Acoustic and behavioural changes by fin whales (Balaenoptera physalus) in response to shipping and airgun noise. Biol. Conserv. 147, 115–122. doi: 10.1016/j.biocon.2011.12.021
Cerchio, S., Andrianantenaina, B., Lindsay, A., Rekdahl, M., Andrianarivelo, N., and Rasoloarijao, T. (2015). Omura’s whales (Balaenoptera omurai) off northwest Madagascar: ecology, behaviour and conservation needs. R. Soc. Open Sci. 2:150301. doi: 10.1098/rsos.150301
Cerchio, S., Andrianantenaina, B., Zerbini, A., Pendleton, D., Rasoloarijao, T., and Cholewiak, D. (2018). Residency, feeding ecology, local movements and potential isolation of the Madagascar Omura’s whale (Balaenoptera omurai) population. Paper SC/67B/NH/09 Presented to the International Whaling Commission Scientific Committee, Histon and Impington, 25.
Cerchio, S., Strindberg, S., Collins, T., Bennett, C., and Rosenbaum, H. (2014). Seismic surveys negatively affect humpback whale singing activity off northern Angola. PLoS One 9:e86464. doi: 10.1371/journal.pone.0086464
Cerchio, S., and Yamada, T. (2018). “Omura’s whale: Balaenoptera omurai,” in Encyclopedia of Marine Mammals, 3rd Edn, eds B. Würsig, J. G. M. Thewissen, and K. Kovacs (San Diego, CA: Academic Press), 656–659. doi: 10.1016/B978-0-12-804327-1.00186-2
Chien, P. V., and Quan, N. V. (2013). “Balaenoptera omurai Wada, Oishi, Yamada, 2003 (Balaenopteridae): a new record for the marine mammals of Vietnam [in Thai],” in Proceedings of the 5th National Conference on Ecology and Biological Resources, Hanoi, 39–43.
Cholewiak, D., Clark, C. W., Ponirakis, D., Frankel, A., Hatch, L. T., Risch, D., et al. (2018). Communicating amidst the noise: modeling the aggregate influence of ambient and vessel noise on baleen whale communication space in a national marine sanctuary. Endanger. Species Res. 36, 59–75. doi: 10.3354/esr00875
Clark, C. W., Ellison, W. T., Southall, B. L., Hatch, L. T., Van Parijs, S. M., Frankel, A., et al. (2009). Acoustic masking in marine ecosystems: intuitions, analysis, and implications. Mar. Ecol. Prog. Ser. 395, 201–222. doi: 10.3354/meps08402
Cypriano-Souza, A. L., Meirelles, A. C. O., Carvalho, V. L., and Bonatto, S. L. (2016). Rare or cryptic? The first report of an Omura’s whale (Balaenoptera omurai) in the South Atlantic Ocean. Mar. Mamm. Sci. 33, 80–95. doi: 10.1111/mms.12348
de Vos, A. (2017). First record of Omura’s whale, Balaenoptera omurai, in Sri Lankan waters. Mar. Biodivers. Rec. 10:18. doi: 10.1186/s41200-017-0121-2
Dolar, M. L., Leatherwood, S., Wood, C. J., Alava, M. N., Hill, C. L., and Aragones, L. V. (1994). Directed fisheries for cetaceans in the Philippines. Rep. Int. Whal. Comm. 44, 439–449.
Egami, T., and Kojima, K. (2017). Records of whaling and societal change in Lamalera, Indonesia. Paper Presentation at 28th Annual Meeting of the Cetology Study Group of Japan, Sapporo.
Erbe, C., Dunlop, R., Jenner, K. C. S., Jenner, M. N., McCauley, R. D., Parnum, I., et al. (2017). Review of underwater and in-air sounds emitted by Australian and antarctic marine mammals. Acoust. Aust. 45, 179–241. doi: 10.1007/s40857-017-0101-z
Forney, K. A., Ferguson, M. C., Becker, E. A., Fiedler, P. C., Redfern, J. V., Barlow, J., et al. (2012). Habitat-based spatial models of cetacean density in the eastern Pacific Ocean. Endanger. Species Res. 16, 113–133. doi: 10.3354/esr00393
Garrigue, C., and Poupon, M. (2013). Guide d’identification: Mammifères Marins de Nouvelle-Calédonie [in French]. Nouméa: Artypo.
Goto, M. (2012). Tsunoshimakujirano gaibukeitaito bunpuikino suitei oyobi Bryde’s whale complexni kansuru ichikousatsu (external morphology and estimated distribution range of Omura’s whale and a consideration on the Bryde’s whale complex.) [in Japanese]. Geiken Tsushin 455, 1–9.
Hamilton, T. A., Redfern, J. V., Barlow, J., Ballance, L. T., Gerrodette, T., Holt, R. S., et al. (2009). Atlas of Cetacean Sightings for Southwest Fisheries Science Center Cetacean and Ecosystem Surveys, 1986-2005. Silver Spring, MD: NOAA, 77.
Ishikawa, H., Goto, M., and Mogoe, T. (2013). Stranding record in Japan 1901-2012 [in Japanese]. Shimonoseki Mar. Sci. Rep. 1, 11–15.
Jung, J. L., Mullié, W. C., Van Waerebeek, K., Wagne, M. M., Bilal, A. S. O., Sidaty, Z. A. O., et al. (2015). Omura’s whale off West Africa: autochthonous population or inter-oceanic vagrant in the Atlantic Ocean? Mar. Biol. Res. 12, 66–75. doi: 10.1080/17451000.2015.1084424
Kahn, B. (2001). Komodo National Park Cetacean Surveys: A Rapid Ecological Assessment of Cetacean Diversity, Abundance and Distribution. Report to the Nature Conservancy Indonesia Coastal and Marine Program. Jakarta: Nature Conservancy Coastal and Marine Program.
Kahn, B. (2005). Indonesia Oceanic Cetacean Program Activity Report: January-February 2005. Report to The Nature Conservancy SE Asia Center for Marine Protected Areas. Cairns: Apex Environmental.
Kahn, B., Wawandono, N. B., and Subijanto, J. (2001). Protecting the Cetaceans of Komodo National Park, Indonesia: Positive Identification of the Rare Pygmy Bryde’s Whale (Balaenoptera edeni) with the Assistance of Genetic Profiling. Final Report to the Indonesian Institute of Sciences. Jakarta: Indonesian Institute of Sciences.
Kawamura, A. (1980). Food habits of the Bryde’s whales taken in the South Pacific and Indian Oceans. Sci. Rep. Whales Res. Inst. 32, 1–23.
Kershaw, F., Leslie, M. S., Collins, T., Mansur, R. M., Smith, B. D., Minton, G., et al. (2013). Population differentiation of two forms of Bryde’s whales in the Indian and Pacific Oceans. J. Hered. 104, 755–764. doi: 10.1093/jhered/est057
Kim, J. H., Kim, H. W., Kim, E. M., and Sohn, H. (2018). First record of the Omura’s whale (Balaenoptera omurai) in Korean waters. Anim. Syst. Evol. Divers. 34, 162–167.
Kondo, I. (2001). Nihon Enganhogeino Kobo (Rise and fall of coastal whaling in Japan) [in Japanese]. Tokyo: San’yo Sha.
Leatherwood, S., and Reeves, R. R. (eds). (1989). Marine Mammal Research and Conservation in Sri Lanka 1985-1986. United Nations Environment Programme (UNEP). UNEP Marine Mammal Technical Report No. 1. Nairobi: UNEP.
LeDuc, R., and Dizon, A. E. (2002). “Reconstructing the rorquals phylogeny: with comments on the use of molecular and morphological data for systematic study,” in Molecular and Cell Biology of Marine Mammals, eds C. Pfeiffer and P. E. Nachtigall (Florida: Krieger), 100–110.
Lei, S., Na, W., Shuai, Z., and Li, G. (2017). Overview on China’s rare earth industry restructuring and regulation reforms. J. Resour. Ecol. 8, 213–222. doi: 10.5814/j.issn.1674-764x.2017.03.001
Ma, M., Zhu, Q., Li, X., Sun, Y., Liu, Y. Y., Liu, Y. N., et al. (2007). Identification of Balaenoptera omurai from an unknown whale vertebra based on Cyt b gene sequence [in Chinese]. Acta Theriol. Sin. 27, 288–292.
Marx, F. G., and Fordyce, R. E. (2015). Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. R. Soc. Open Sci. 2:140434. doi: 10.1098/rsos.140434)
McCauley, R. D. (2009). Sea Noise Logger Deployment Scott Reef, 2006-2008 – Whales, Fish and Seismic Surveys. Report prepared for Woodside Energy, CMST R2009-15. 88.
McCauley, R. D. (2014). Joseph Bonaparte Gulf Sea Noise Logger Program, Sep-2010 to Sep-2013, Ambient Noise, Great Whales and Fish. Report prepared for RPS MetOcean, CMST R2013-52, 75.
McPherson, C., Kowarski, K., Delarue, J., Whitt, C., MacDonnell, J., and Martin, B. (2016a). Passive Acoustic Monitoring of Ambient Noise and Marine Mammals—Barossa Field: JASCO Document 00997, Version 1.0. Technical report by JASCO Applied Sciences for Jacobs. Capalaba: JASCO.
McPherson, C. R., Martin, B., MacDonnell, J., and Whitt, C. (2016b). Examining the value of the Acoustic variability index in the characterisation of Australian marine soundscapes. Proc. Acoust. 2016, 9–11.
Miyakawa, N. (2018). “A record of Omura’s whale stranding on Katsuura coast, in July 2017,” in Proceedings of the 2018 Annual Meeting of the Mammal Society of Japan, Nagano.
Moreira, S. C., Sousa-Lima, R. S., Maia, M., Sukhovich, A., Royer, J. Y., and Cerchio, S. (2018). “Caracterização das emissões sonoras de Balaenoptera omurai no Oceano Atlântico equatorial: Uma contribuição para melhor avaliação da distribuição da espécie [in Portuguese],” in Proceedings of the Abstracts XII Congreso de la Sociedad Latinoamericana de Especialistas en Mamíferos Acuáticos – RT 18, Lima, 231.
Mustika, P. T. K. (2006). Marine Mammals in the Savu Sea (Indonesia): Indigenous Knowledge, Threat Analysis and Management Options. M.Sc. thesis, James Cook University, Townsville, 231.
Notarbartolo di Sciara, G., Kerem, D., Smeenk, C., Rudolph, P., Cesario, A., Costa, M., et al. (2017). Cetaceans of the red Sea. CMS Tech. Ser. 33:86.
Nowacek, D. P., Thorne, L. H., Johnston, D. W., and Tyack, P. L. (2007). Responses of cetaceans to anthropogenic noise. Mamm. Rev. 37, 81–115. doi: 10.1111/j.1365-2907.2007.00104.x
Ohsumi, S. (1980). Population study of the Bryde’s whale in the southern hemisphere under scientific permit in the three seasons 1976/77–1978/79. Rep. Int. Whal. Comm. 30, 319–331.
Oishi, M., Wada, S., and Yamada, T. K. (2004). Outline of the research on Tsunoshima-kujira, Balaenoptera omurai, with the comments on the classification of so-called Bryde’s whales, B. edeni and B. brydei [in Japanese]. Nihonkai Cetol. 14, 1–12.
Omura, H. (1962). Further information on Bryde’s whale from the coast of Japan. Sci. Rep. Whales Res. Inst. 16, 7–18.
Omura, H. (1977). Review of the occurrence of Bryde’s whale in the northwest Pacific. Rep. Int. Whal. Comm. 1, 88–91.
Oremus, M., Leqata, J., Hurutarau, J., Taei, S., Donoghue, M., Thompson, K., et al. (2011). Solomon Islands Dolphin Project: Progress Report on Data Collection and Analyses. Technical Report to the South Pacific Whale Research Consortium. Honiara: South Pacific Whale Research Consortium, 27.
Ottewell, K., Coughran, D., Gall, M., Irvine, L., and Byrne, M. (2016). Stranding of Omura’s Whale (Balaenoptera omurai) in Western Australia. Aquat. Mamm. 42, 193–197. doi: 10.1578/AM.42.2.2016.193
Palmer, M. A., Bernhardt, E. S., Schlesinger, W. H., Eshleman, K. N., Foufoula-Georgiou, E., Hendryx, M. S., et al. (2010). Mountaintop mining consequences. Science 327, 148–149. doi: 10.1126/science.1180543
Perrin, W. F. (2007). The Philippine fishery for Bryde’s whales in the Western North Pacific, 1983-1986. Paper SC/58/RMP1 Presented to the International Whaling Commission Scientific Committee, Cambridge.
Perrin, W. F., Dolar, M. L. L., and Ortega, E. (1996). Osteological comparison of Bryde’s whales from the Philippines with specimens from other regions. Paper SC/47/NP2 Presented to the International Whaling Commission Scientific Committee, Cambridge.
Ponnampalam, L. S. (2012). Opportunistic observations on the distribution of cetaceans in the Malaysian South China, Sulu and Sulawesi Seas and an updated checklist of marine mammals in Malaysia. Raffles B. Zool. 60, 221–231.
Ranjbar, S., Dakhteh, S. M. H., and Van Waerebeek, K. (2016). Omura’s whale Balaenoptera omurai stranding on Qeshm Island, Iran, Persian Gulf: further evidence for a wide (sub)tropical distribution. J. Mar. Biol. Oceanogr. 5:3. doi: 10.4172/2324-8661.1000161
Rosel, P. E., and Wilcox, L. A. (2014). Genetic evidence reveals a unique lineage of Bryde’s whales in the northern Gulf of Mexico. Endanger. Species Res. 25, 19–34. doi: 10.3354/esr00606
Sasaki, T., Nikaido, M., Wada, S., Yamada, T. K., Cao, Y., Hasegawa, M., et al. (2006). Balaenoptera omurai is a newly discovered baleen whale that represents an ancient evolutionary lineage. Mol. Phylogenet. Evol. 41, 40–52. doi: 10.1016/j.ympev.2006.03.032
Slater, G. J., Goldbogen, J. A., and Pyenson, N. D. (2017). Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proc. R. Soc. B 284:20170546. doi: 10.1098/rspb.2017.0546
Sousa, A. G., and Harris, D. (2015). Description and seasonal detection of two potential whale calls recorded in the Indian Ocean. J. Acoust. Soc. Am. 138, 1379–1388. doi: 10.1121/1.4928719
Thomas, P. O., Reeves, R. R., and Brownell, R. L. (2015). Status of the world’s baleen whales. Mar. Mamm. Sci. 32, 682–734. doi: 10.1111/mms.12281
Vahidi, E., Navarro, J., and Zhao, F. (2016). An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour. Conserv. Recy. 113, 1–11. doi: 10.1016/j.resconrec.2016.05.006
Van Canneyt, O., Dorémus, G., Laran, S., Ridoux, V., and Watremez, P. (2015). REMMOA Nouvelle-Calédonie Wallis et Futuna: Rapport de campagne. Rapport intermédiaire pour l’Agence des Aires Marines Protégées. Brest: ENSTA Bretagne, 65.
Wada, S., and Numachi, K. (1991). Allozyme analyses of genetic differentiation among the populations and species of the Balaenoptera. Rep. Int. Whal. Commn. 13, 125–154.
Wada, S., Oishi, M., and Yamada, T. K. (2003). A newly discovered species of living baleen whale. Nature 426, 278–281. doi: 10.1038/nature02103
Wang, H., Fan, Z., Shen, H., and Peng, Y. (2006). Description of new record species of whales from Chinese coastal waters [in Chinese]. Fisheries Sci. 25, 85–87. doi: 10.3969/j.issn.1003-1111.2006.02.010
Weber, M. W. C. (1923). Die Cetaceen der Siboga-Expedition [in German]: Vorkommen und Fang der Cetaceen im Indo-Australischen Archipel. Dordrecht: Brill.
Wilmet, L., Devillers, P., and Beudels-Jamar, R. C. (2017). Priceless ecosystems threatened by a rare earth mining project in northwest Madagascar – Ampasindava Peninsula. Lemur News 20, 3–4.
Wilson, S., Meekan, M., Carleton, J., Stewart, T., and Knott, B. (2003). Distribution, abundance and reproductive biology of Pseudeuphausia latifrons and other euphausiids on the southern North West Shelf, Western Australia. Mar. Biol. 142, 369–379. doi: 10.1007/s00227-002-0945-z
Xu, M., Wang, X., Miao, X., Wu, F., Ma, M., Tao, C., et al. (2017). A stranding of Omura’s whale Balaenoptera omurai Wada, Oishi and Yamada, 2003) in the Taiwan Strait, China. Aquat. Mamm. 43, 289–298. doi: 10.1578/AM.43.3.2017.289
Yamada, T. K. (2009). “Omura’s whale, Balaenoptera omurai,” in The Wild Mammals of Japan, eds S. Ohdachi, Y. Ishibashi, M. Iwasa, and T. Saitoh (Kyoto: Shoukahoh Book Sellers and Mammalogical Society of Japan), 330–331.
Yamada, T. K., Chou, L. S., Chantrapornsyl, S., Adulyanukosol, K., Chakravarti, S. K., Oishi, M., et al. (2006a). Middle-sized balaenopterid whale specimens (Cetacea: Balaenopteridae) preserved at several institutions in Taiwan, Thailand, and India. Mem. Natl. Sci. 44, 1–10.
Yamada, T. K., Kemper, C., Tajima, Y., Umetani, A., Janetzki, H., and Pemberton, D. (2006b). Marine mammal collections in Australia. Natl. Sci. Museum Monogr. 34, 117–126.
Yamada, T. K., Kakuda, T., and Tajima, Y. (2008). Middle sized balaenopterid whale specimens in the Philippines and Indonesia. Mem. Natl. Sci. 45, 75–83.
Yang, G., Liu, H., Zhou, K., and Ji, G. (2002). Identification of a Balaenoptera edeni specimen by using mitochondrial DNA sequences [in Chinese]. Chin. J. Zool. 37, 35–38.
Yang, X. J., Lin, A., Li, X., Wu, Y., Zhou, W., and Chen, Z. (2013). China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 8, 131–136. doi: 10.1016/j.envdev.2013.03.006
Keywords: Omura’s whale, Balaenoptera omurai, distribution, genetic identification, photographic identification, acoustics, threats, conservation
Citation: Cerchio S, Yamada TK and Brownell RL Jr (2019) Global Distribution of Omura’s Whales (Balaenoptera omurai) and Assessment of Range-Wide Threats. Front. Mar. Sci. 6:67. doi: 10.3389/fmars.2019.00067
Received: 01 October 2018; Accepted: 06 February 2019;
Published: 15 March 2019.
Edited by:
Rob Harcourt, Macquarie University, AustraliaReviewed by:
Asha de Vos, The Sri Lankan Blue Whale Project and Oceanswell, Sri LankaMatthew Steven Leslie, Swarthmore College, United States
Copyright © 2019 Cerchio, Yamada and Brownell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salvatore Cerchio, scerchio@neaq.org; scerchio@gmail.com
 Salvatore Cerchio
Salvatore Cerchio Tadasu K. Yamada
Tadasu K. Yamada Robert L. Brownell Jr.
Robert L. Brownell Jr.