Abstract
We examined the reproductive behavior (courtship and mating), seasonality and its distribution in three Mobula species, spinetail, bentfin, and Munk’s devil rays (M. mobular, M. thurstoni, and M. munkiana) in the southwestern Gulf of California, Mexico, using boat surveys (with drone and in-water observations) (n = 69 survey days), spotter planes (n = 428 flights), and citizen science observations (n = 31). We examined whether (1) reproductive grounds existed within the area for any of these species, (2) whether reproductive behavior followed seasonal patterns, and (3) if this behavior was similar among all mobula rays. We observed reproductive behavior in 221 events in 2017 and 2021–2022, for M. mobular (n = 10), M. thurstoni (n = 3), and M. munkiana (n = 208) dispersed along 312 km of the eastern Baja California Peninsula between 4 m and 6.3 km away from the coast. Most events (n = 209) occurred in the La Ventana and Ensenada de Muertos areas. Courtship was observed for M. mobular and M. thurstoni and a copulation attempt for M. munkiana, with reproductive behavior following a seasonal pattern occurring from March to August, with a peak during May (81.9% of the events). Mobula munkiana displayed previously undescribed behaviors, such as the “piggyback leaps” as a pre-copulatory position and the “courtship vortex”, where 122 individuals were observed circling in a clockwise direction for 5 h with courtship groups joining and leaving the main vortex formation. This study highlights the areas of La Ventana and Ensenada de Muertos as critical habitats for reproductive behavior of two endangered and one vulnerable devil ray species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive behavior of many species of sharks and rays (elasmobranchs) is poorly understood due to the difficulties associated with observing courtship and mating events in the wild (Pratt and Carrier 2001). Much of the research on elasmobranch reproductive behavior has been conducted using fresh fisheries’ carcasses (Serrano-López et al. 2021) where reproductive behavior is inferred from mating scars on females or the state of male claspers (Marshall and Bennett 2010; Rangel et al. 2022; Whitehead et al. 2022), or from sporadic observations in the wild (Whitney et al. 2010; Arnés-Urgellés et al. 2018) or captivity (Uchida et al. 1990; Manual 2004; Smith et al. 2004). A few studies report specific reproductive grounds based on direct and repeated observations of courtship and mating behavior in the field with exceptions including whitetip reef shark Triaenodon obesus, reef manta rays M. Alfredi, and basking sharks Cetorhinus maximus (Whitney et al. 2004; Marshall and Bennett 2010; Deakos 2011; Stevens et al. 2018a; Sims et al. 2022). It is particularly important to identify the timing and location of elasmobranch reproductive behavior as such areas may be critical in providing specific conditions that support reproductive success. These areas may be important for elasmobranch conservation and management measures to ensure the viability of future elasmobranch populations (Hyde et al. 2022; Palacios et al. 2023).
Mobulid rays (manta and devil rays) are filter feeding batoids distributed in all oceans from temperate to tropical waters (Couturier et al. 2012; Stevens et al. 2018b). The Mobulidae family has the lowest fecundity of all elasmobranchs (Stevens et al. 2000; Dulvy et al. 2014), giving birth to just one pup per gestation period that lasts up to 13 months (Deakos 2011; Marshall and Bennett 2010; Stevens 2016; Broadhurst et al. 2019). Their reproductive cycles normally involve resting periods, with a 2 to 7 year interval between pregnancies (Deakos 2011; Marshall and Bennett 2010; Stevens 2016). The reproductive strategy of mobulids is aplacental viviparity with histotrophy (Serrano-López et al. 2021) and mate through internal fertilization (Conrath and Musick 2012) occurring after courtship behaviors that can last from several minutes to days (Marshall and Bennett 2010; Stevens et al. 2018a). Studies of the reproductive behavior (courtship and mating) of mobulids have focused on a limited number of species such as the oceanic manta ray M. birostris, M. alfredi, and sicklefin devil ray M. tarapacana (Marshall and Bennett 2010; Deakos 2011; Stevens et al. 2018a, b; Mendonça et al. 2020) with anecdotical observations of other mobulid species (spinetail devil ray M. mobular, bentfin devil ray M. thurstoni, Atlantic pygmy devil ray M. hypostoma, and shorthorned pygmy devil ray M. kuhlii) (Coles 1910; Duffy and Tindale 2018; McCallister et al. 2020; Carpenter and Griffiths 2023). These studies examined all or some of the seven stages described for mobulid courtship and mating (Stevens 2016; Stevens et al. 2018a). Courtship behavior includes the first four stages: initiation, endurance, evasion, and pre-copulatory positioning, while mating behavior refers to the three stages when copulation, post-copulation holding, and separation occurs (Stevens et al. 2018a). During courtship events, several individuals are involved, with one or two females chased by males, in a formation described as a courtship train, numbering from just a few to up to 26 males (Marshall and Bennett 2010; Stevens et al. 2018a). Mobulid courtship aggregations have been described at: oceanic islands, seamounts, ridge systems, coral reefs, feeding aggregation sites, and cleaning stations (Yano et al. 1999; Marshall and Bennett 2010; Sobral 2013; Stevens et al. 2018a; Germanov et al. 2019; Mendonça et al. 2020; Palacios et al. 2023). While reproductive behavior has been described for the larger mobulids (e.g., M. birostris and M. alfredi), there are only two observational descriptions of courtship behaviors for the pygmy devil ray (M. hypostoma and M. kuhlii) (Coles 1910; Carpenter and Griffiths 2023) with only one mating event described (M. hypostoma) (Coles 1910). Lack of information currently exists on how or where these devil ray species mate or whether they follow the same courtship behaviors described for the better-studied species (Childs 2001; Notarbartolo-di-Sciara et al. 2019). In this study, we use direct field observations to examine the courtship and mating behavior of three lesser known devil ray species: M. mobular, M. thurstoni, and M. munkiana.
Due to their conservative life-history traits (Dulvy et al. 2014) and anthropogenic threats, including target fisheries and bycatch (Croll et al. 2016; Lezama-Ochoa et al. 2019), all mobulids species are listed as Endangered or Vulnerable on the IUCN’s Red List of Threatened Species (IUCN 2020). In the Mexican Pacific, the most abundant devil ray species are M. mobular, M. thurstoni, and M. munkiana (Notarbartolo-di-Sciara G, 1988; Serrano-López et al. 2021). Mobulids have been protected in Mexico since 2006 by NOM-029-PESC-2006, and since 2019 by NOM-059-SEMARNAT-2010 regulations, with their primary threats including incidental capture in artisanal fisheries using gillnets (Del-Valle-González-González 2018) as well as bycatch in industrial fisheries, especially in the tuna purse seine fishery (Croll et al. 2012, 2016; Lezama-Ochoa et al. 2019). In recent years, emerging nonregulated ecotourism activities based on snorkeling with mobulid aggregations have brought new economic opportunities to the local communities in the southern Gulf of California, Mexico. However, increasing ecotourism, particularly during critical life-history stages and at key aggregation sites (e.g., mating, nursery), could potentially become a problematic source of disturbance (Murray et al. 2020).
Mobula mobular and M. thurstoni are found globally in tropical and subtropical oceans, and can reach up to 3.20 m and 1.83 m disc width (DW), respectively (Stevens et al. 2018b; Stewart et al. 2018). Courtship for these species has been reported from direct observations at offshore areas of New Zealand (M. mobular, Duffy and Tindale 2018) and in Brazil (M. thurstoni, McCallister et al. 2020). In the southern Gulf of California, Mexico, M. thurstoni is present year-round (Notarbartolo-di-Sciara G, 1988; Serrano-López et al. 2021), while M. mobular is present April–August and October–December (Notarbartolo-di-Sciara G, 1988; Croll et al. 2012; Serrano-López et al. 2021). The reproductive season for these species occurs during June and July, inferred from morphometry and histology studies of fisheries caught individuals in this region (Notarbartolo-di-Sciara G, 1988; Serrano-López et al. 2021). Mobula munkiana is distributed in the eastern tropical Pacific, is present year-round in the southern Gulf of California and reaches a maximum DW of 1.30 m (Stewart et al. 2018; Serrano-López et al. 2021; Palacios et al. 2021). No direct observations of M. munkiana reproductive behavior have been reported for this species; however, Palacios et al. (2021) speculated that they mate in the southern Gulf of California from April to June, based on courtship observations and the presence of sperm in the developed claspers of males captured at the Espiritu Santo Archipelago.
Diver avoidance behavior by devil rays and a lack of survey effort focused on these three devil ray species has resulted in significant knowledge gaps in important life-history parameters and behaviors, particularly reproduction. However, recent citizen science efforts, collaborations with tourism companies using spotter seaplanes, and the use of scientific drones have facilitated field observations in remote or inaccessible areas (Stevens et al. 2018a; Ehemann et al. 2022; Rambahiniarison et al. 2022) and the collection of important behavioral and demographic data on mobulid species (Setyawan et al. 2020, 2022).
Here, we examined the behavior, distribution, and seasonality of reproductive events for M. mobular, M. thurstoni, and M. munkiana in the southern region of the Gulf of California, Mexico, to determinate (1) if reproductive grounds exist within the Gulf of California area for any of these species, (2) if reproductive behavior of the three species follows a seasonal pattern, and (3) if courtship and mating behavior is similar among mobulid species.
Methods
Study area
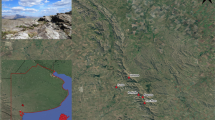
All reproductive behavior observations described in this study occurred in the southern region of the Gulf of California, along the east coast of the Baja California Peninsula, Mexico (Fig. 1, a, b). The area is characterized by sandy and rocky coastlines with deep depths (> 3700 m), small tidal ranges (annual maximum range of 2.3 m), and sea surface temperatures ranging between 20 and 30 ºC (Brusca et al. 2005). The ocean productivity in the area is influenced by a monsoonal wind pattern, with northwesterly winds causing upwelling events during the cold season (December–May), with an average primary production of 10 mg. m–3 of sea surface chlorophyll a (Santamaria-Del-Angel et al 1999; Lavin and Marinone 2003). During the warm season (June–November), strong thermal stratification occurs, with warm water from the Pacific entering the southern Gulf of California, and southeast winds create weak upwelling on the peninsula east coast with an average primary production of 0.1 mg. m–3 (Santamaría-del-Ángel et al. 1999, Lavín and Marinone 2003).
(a) Location of Baja California Peninsula in Mexico (b) Study area in the southwestern region of the Gulf of California. Black polygons indicate MPAs in the region: Cabo San Lucas (CSL), Cabo Pulmo (CP), Espíritu Santo Archipelago (ESA) and Bahía de Loreto (BL). Orange and yellow polygons are the areas surveyed by seaplanes, the red polygon is the area covered by boat surveys near Cerralvo Island (CI), and the black points are sightings reported by citizen scientists. (c) Survey effort (number of surveys, left axis) during 2021–2022 for two seaplanes, boat surveys, and sightings days from citizen scientists (colors correspond to the legend in panel b), and the number of reproductive events (right axis) observed (blue cross x). (d) Effort-corrected reproductive events by two seaplanes (orange line) and boat surveys (red bars) from March to August
Data collection
Behavioral data
Reproductive behavior refers to courtship and mating events and was distinguished from other behaviors such as feeding and cruising following the criteria proposed by Stevens et al. (2018a) for mobulids. We define breaching behavior as an arial behavior where individual mobulids accelerate rapidly towards the surface, propelling themselves clear of the water (Medeiros et al. 2021).
Near-term pregnancy was identified by the distended abdominal area on the dorsal and ventral surfaces in females (Marshall and Bennett 2010; Stevens et al. 2018a). We confirmed the external evidence of late-term pregnancy using ultrasound on M. munkiana individuals (Palacios unpubl data). Maturity was stablished based on visual estimation of body size or elongated claspers beyond their pelvic fins (males) and presence of mating scars or wounds (females). Mating scars and wounds were identified as the parallel wound scratches and abrasions healed (mating scars) or fresh (mating wounds) on females' left pectoral fins (dorsal or ventral) resulting from the teeth of conspecific males to hold her fin during copulation (Stevens 2016; Stevens et al. 2018a). Mating scars are evidences of past mating events (months or years), while mating wounds are evidences of recent mating events (days or weeks) (Stevens 2016; Stevens et al. 2018a).
Boat surveys
Between May 2021 and June 2022, a total of 69 survey days (between 2 to 24 surveyed days per month) were conducted in La Ventana and Ensenada de Muertos area (Fig. 1b). Each survey consisted of a non-systematic transect of at least 5 h (7 am–12 pm) of observations from a small boat (panga) covering a fixed study area (Fig. 1b) during conditions of Beaufort sea state ≤ 2. Once an individual or mobulid group were located by sight (by their breaching behavior and/or swimming activity at or near the surface), the research boat remained ~ 20-m distant from the animals with the motor switched off or in neutral to record group information. A drone (DJI Mavic Pro 2) was launched from the boat and aerial observations were conducted flying at an altitude between 20 and 50 m above ocean surface for 10-20 min. Finally, when possible, in-water observations were conducted by free divers taking video and/or still images using a GoPro7. For each sighting, we recorded date, time, location, species observed and estimated number of individuals, behavior, and, when possible, pregnancy status and maturity stage.
Citizen science data
Photographs and video files of mobulid reproductive events were collected from ecotourism guides and ecotourism boat captains in Baja California Sur (from La Paz to Cabo San Lucas, along the western Gulf of California coast) during 2017, 2021, and 2022. Images were elicited during public educational talks with local communities and submitted via email with information on the sighting date and location. All reported sightings of M. mobular and M. thurstoni were accompanied by photographic material to verify the accuracy of the data. Mobula munkiana sightings from citizen scientists that did not have associated photographic evidence were only considered if provided by guides trained (n = 5) in the collection of these data. These guides were trained on species identification and behavior recognition with the lead author of this study. All video and photographic materials are used with permission and include owner credit.
Seaplane data
During 2021–2022, spotter flights operated by private tourism companies were conducted on the southeastern portion of the Gulf of California, originating from La Paz, Baja California Sur. Although these flights did not follow a standardized transect, they were designed to spot megafauna in the area and all sightings of marine animals were recorded during the flight by trained observers with location, date, species, number of individuals or size of the group and, behavior. A total of 428 flights were conducted, each flight lasted between 2 and 4 h for a total of 858.3 flight hours between January 2021 and December 2022. The total study area covered by these flights was 8317 km2, and flights were conducted during all months of the year (Fig. 1b Seaplane I). During May–August 2022, seven flights covered an additional area of 8547 km2 along the coast from Cabo San Lucas to Loreto (Fig. 1b Seaplane II). The weather conditions during surveys were Beaufort sea state ≤ 3, no clouds, with light and gentle breeze. The aircraft used for these flights was an SLA Seaplane or a Citabria flying at an altitude of ~ 500–1000 feet (152–305 m) at a ground speed between ~ 45 and 69 knots (83–128 km–1). During these surveys, maturity and sex of mobula individuals were assumed based on ongoing behavior (courtship trains).
Analysis
Individuals observed in videos and photographs were counted using the software ImageJ (ImageJ) using the multipoint tool. To estimate the number of reproductive events observations per effort, we divided the number of observations per week (from boat surveys) and per month (from seaplane observations) between the days at sea for each method. Maps were created using Surface Mapping System (Golden Software, Inc., https://www.goldensoftware.com/products/surfer) and the coastline data were extracted from GEODASNG (National Geophysical Data Center, 2000).
Results
A total of 221 direct observations of reproductive behavior were recorded between March and August in 2017 (n = 1, 0.5%), 2021 (n = 13, 6%), and 2022 (n = 207, 93.5%) (Supplementary material Table 1). We recorded 126 (57%) courtship events by seaplane, 64 (29%) by boat surveys, and 31 (14%) by citizen science images (Table 1). The single copulation attempt event we observed for M. munkiana was recorded during boat surveys using the drone. All reproductive events were observed between the surface and ~ 5 m depth.
Distribution and seasonality of reproductive behaviors
All reproductive behaviors events occurred during spring and summer months (Fig. 2), coinciding with the transition between the cold season (December to May) and warm season (June to November) in the southern Gulf of California. The peak of reproductive events occurred during the month of May.
Seasonality of M. mobular, M. thurstoni and M. munkiana reproductive behavior in the southern Gulf of California based on fisheries information from previous research (Notarbartolo-di-Sciara 1988; Guerrero-Maldonado 2002; Serrano-Lopez et al. 2021) and from direct observations reported on this study. Red indicates warm water months, while blue indicates cold water months. White colored months indicate absence of the species in the study area (Notarbartolo-di-Sciara 1988; Serrano-Lopez et al. 2021). Black crosses (x) indicate courtship and mating from the literature, and white crosses (x) indicate courtship and mating observations in the present study. Devil ray species illustrations by Julie Johnson, Life Science Studio
Sightings were dispersed along 312 km of the east coast of the Baja California Peninsula, from Cabo San Lucas to Ensenada de Cortes at the entrance of San Jose Island channel between the peninsula coast and San Jose Island (SJI); however, the majority (n = 209, 95%) occurred within La Ventana (LV) and Ensenada de Muertos (EM) areas (Fig. 3). Observations occurred between 4 m and 6.3 km of the coast, while the plane flew from 0 to 60 km offshore.
(a) Southern portion of Baja California Peninsula, with reproductive behavioral events (colored dots) observed between 2017 and 2022 for M. mobular (green), M. thurstoni (yellow), and M. munkiana (red). Black polygons indicate MPAs in the region: CSL, CP, and ESA. (b) Expanded detail in the area of La Ventana (LV), Ensenada de Muertos (EM), and Cerralvo Island (CI) as indicated by the yellow polygon in (a) Event 7: On 20 May 2021 at the Espiritu Santo Archipelago (Fig. 4 e–h), one near-term pregnant individual, was chased by three males (endurance) (Fig. 4e), one of the males subsequently positioned himself on top of the female’s dorsal surface attempting to reach and bite the left pectoral fin of the female (pre-copulation positioning) (Fig. 4f–g). After two failed attempts, the female performed four forward somersaults copied by the same male (evasion) (Fig. 4h)
Mobula mobular
Courtship behavior was observed for M. mobular on 10 events (Table 1) during the months of May (n = 5) in 2017, 2021, 2022, July (n = 4) in 2022, and August (n = 1) in 2022. Sightings were dispersed more than 312 km along the east coast of Baja California Peninsula, from Cabo San Lucas to Ensenada de Cortes at the northern entrance of San Jose Island channel (Fig. 3a). During a courtship event observed in May 2021, one of the females was pregnant in the last stages of gestation (evidenced by a highly distended abdomen).
Mobula thurstoni
Courtship behavior was observed for M. thurstoni on three events (Table 1) during the months of June (n = 1) in 2021, July (n = 1) in 2022, and August (n = 1) in 2022. Sightings were dispersed more than 43 km along the east coast of Baja California Peninsula, from El Saltito to Punta Arenas (Fig. 3b).
Mobula munkiana
Reproductive behavior was observed for M. munkiana on 208 events (Table 1) during the months of March (n = 4) in 2022, April (n = 5) in 2022, May (n = 177), June (n = 16) and July (n = 3) during 2021–2022, and finally in August (n = 3) in 2022. The peak of sightings was during the month of May in both years. During this peak, increased courtship activity was observed for the last 10 days of the month. Sightings were dispersed more than 184 km along the east coast of Baja California Peninsula, from El Saladito in the Bay of La Paz to Cabo Pulmo (Fig. 3). A copulation attempt was observed (n = 1) during a boat survey (28 May 2022) and filmed with the drone. In addition, the following day during a boat survey (29 May 2022), a large number of courtships trains were observed in La Ventana over an extended area. A seaplane flight flying in a straight transect of 8.06 km (Supplementary material Fig. 1) in the area counted 102 courtship trains on 29 May 2022.
Reproductive behavior description
Here, we provide detailed descriptions of the reproductive behaviors of these species.
Mobula mobular
The number of individuals involved in the documented M. mobular courtship events (n = 10) ranged from two to nine, with the sex of individuals determined for three of these events (recorded by citizen science observations) (Fig. 4). Three of the seven stages of courtship and mating behavior (Stevens et al. 2018a) were recorded. Detailed descriptions of events are provided below.
Event 1: On 5 May 2017 at Cabo San Lucas (Fig. 4a–d), a group of nine individuals (two females and seven males) were observed engaging in courtship behavior. One of the females was chased by seven males at high-speed close to the surface (endurance) (Fig. 4a), with one of the males approaching the female by the ventral side (Fig. 4b). The female performed one forward somersault, copied by two males (evasion) (Fig. 4c) and one minute and 10 s later reduced speed, and stopped at the surface (pre-copulation positioning), while one of the males approached her from underneath (Fig. 4d). Right after the female swam down followed by one male and the observations stopped.
Event 8: Another event was observed in proximity and on the same day (Fig. 4i,zj). It involved one female and one male performing erratic movements and forward somersaults (evasion) (Fig. 4i) and the positioning of the male on top of the female, trying to reach the female’s left pectoral fin (pre-copulation positioning) (Fig. 4j).
Mobula thurstoni
The number of individuals in M. thurstoni courtship groups ranged between 3 and 5, although courtship occurred within bigger groups (> 10) that included individuals not engaging in reproductive behavior. Three of the seven stages of courtship and mating behavior (Stevens et al. 2018a) were recorded for M. thurstoni. Detailed descriptions of events are provided below.
Event 209: On 28 June 2021 at El Saltito Beach (Fig. 5a–d), a group of five individuals (one female and four males) were engaged in courtship behavior at the surface. A female with mating scars on her left pectoral fin was chased at speeds elevated above average swimming speed by three males (endurance) (Fig. 5a–b). The female performed erratic movements, changes of direction, and somersaults (evasion) (Fig. 5c). During these movements, the female was copied by a male directly behind her during the entire observation period. This male approached the female from underneath, and then positioned himself on top of the female (pre-copulation positioning) (Fig. 5d).

© Adriá Bosch-Soler. Event 210: (e) endurance, (f, g, h) evasion, and (g) pre-copulation positioning. Photo © Maru Brito. Event 217: (i) evasion and (j) pre-copulation positioning. Photo © Afelandra González-Cibrián
Mobula thurstoni courtship events. Event 209: (a, b) endurance, (c) evasion, (d) pre-copulation positioning. Photo
Event 210: On the 1 July 2022 at Punta Arenas (Fig. 5e–h), a female with fresh mating wounds on her left pectoral fin was chased by three males in a courtship train (endurance) (Fig. 5e), while performing turns (evasion) (Fig. 5, f). The third male in the train advanced position and speed to a position on top of the female’s dorsal surface (pre-copulation positioning) (Fig. 5g–h).
Event 217: On the 5 August 2022 in La Ventana (Fig. 5i–j) within a group of M. thurstoni (> 10), a courtship train of two males was recorded chasing a female (endurance) (Fig. 5i) and performing backward somersaults (evasion) (Fig. 5j).
Mobula munkiana
We observed a maximum of 29 M. munkiana individuals in a single reproductive group. We recorded five of the seven stages of reproductive behavior (Stevens et al. 2018a) in M. munkiana. Below, we present descriptions of two previously undocumented reproductive behaviors for mobulids, the courtship vortex, and the piggyback leaping (pre-copulatory behavior) enhancing and broadening our comprehension of pygmy devil ray reproductive behaviors.
Event 6: On 19 May 2021 at Ensenada de Muertos (Fig. 6a–c), a vortex formation at the surface between 2 and 5 m wide was observed during a 5-h period starting the observation at 9:40 am (Supplementary material, Video 1). During this event, females and males (n = 122) were swimming at a consistent average swimming speed, circling in a clockwise direction, collectively swimming up and down to a maximum of 5 m depth in the water column. No feeding was observed, yet numerous small courtship trains (approximately 20) where a female was chased by one-to-three males regularly occurred (Fig. 6a). The individuals in courtship trains did not separate more than 5–10 m from the main formation (Fig. 6b–c), returning to the courtship vortex after a few seconds of chase (endurance). Sexually mature males engaged in these courtship trains. No visible pregnant females were observed within the group. Some females presented abrasions on the lower area of their dorsal surface probably produced by the pre-copulatory positioning of the male on top of them (Fig.7a-d). Breaching behavior was not observed in the vortex during the 5-h observation period.

© Shawn Hendrich and Jay Clue. Event 111: (d) endurance. Photo © Marta D. Palacios. Events 85–186: (e) view of courtship trains from the seaplane in a circle. Photo © Sidharta Velázquez-Hernández. Event 84: (f) Evasion, (g, h) pre-copulation positioning, and (i) copulation attempt. Photo © Marta D. Palacios
Mobula munkiana courtship and mating event. Event 6: (a) endurance and (b, c) courtship vortex. Photo

© Laurent Ballesta. (d) Courtship abrasions on the posterior half of the disc on female’s dorsal side indicated by white arrows (May 2022). Photo © Marta D. Palacios
Reproductive behavior of M. munkiana: piggyback leaping. (a) Male positioning himself onto the female dorsal surface (event 82). (b, c) Examples of males erecting their tails when on top of the female (events 82 and 72). Photos
Events 87–188: On 29 May 2022 at La Ventana (Fig. 6d–e), 102 courtship groups were observed at the surface between 7:24 and 9:10 am. Reproductive behavior consisted of courtship trains (initiation, endurance, and evasion) dispersed within La Ventana area in a straight transect of 8.06 km (Supplementary material, Fig. 1). Courtship groups occurred at the surface, while larger groups (> 100 individuals) of M. munkiana remained cruising a few meters below. On many occasions, several courtship events occurred in proximity. Courtship trains were dynamic with males switching chasing one female to another nearby female.
Event 84: On 28 May 2022 at La Ventana (Fig. 6f–i), a group of 29 individuals (one female and 28 males) were engaging in courtship behavior (Supplementary material, Video 2). The group was observed for 45 min and included one female swimming at below average cruising speed at the surface with her pectoral fins often lifting out of the water. The males (28) were circling, chasing her, and swimming from behind positioning themselves on top of her (piggyback leaping) as a pre-copulatory behavior (Fig. 7), approximately 135 times during the observation period. The female swim speed increased when a male positioned himself directly behind or onto her dorsal surface, turning her swim angle acutely or flipping forwards (evasion). After successfully evading the male, the female returns to the surface and resumes below average swim speeds, slowly moving around within a small area (approx. 50 m2).
Although individual males could not be identified, preventing from an assessment of the number of different males which chased the female, some male individuals chased each other away when in proximity to the female (Supplementary material, Video 3). On one occasion, one male approached the female from underneath while up to three males rushed from behind and swam on top of her (piggyback leaping) (Fig. 6g). However, the most common behavior was when males, one at a time, approached the female from the ventral part and then slid along one of the female's pectoral fins and swam on top of her (piggyback leaping). When on top of the female, males erected their tail dorsally at 90 º (Fig. 7b,c), bending their pelvic area and making rapid pelvic thrusts (Supplementary material, Video 4). On one occasion, we observed a clear copulation attempt where the male swam on top of the female and started to make rapid pelvic thrusts, they both subsequently sank towards the bottom while spinning around (copulation attempt) for 14 s. The pair then separated, and the female swam off to the surface (Supplementary material, Video 2). Immediately after, courtship behavior continued with this female and other males in the group.
Associated observations
Pregnancies
Mobula mobular (n = 1) (event 7) (Fig. 7a) and M. munkiana (= 10) (events 5, 11, 12, 27, 28, 50, 62, 73, 205, 206) females showing evidence of later term were observed engaging in courtship behaviors during the months of May and June 2021–2022.
Mating scars, fresh mating wounds, and courtship abrasions
Parallel fresh mating wound scratches and abrasion on females' left pectoral fins (dorsal or ventral) resulting from the teeth of conspecific males were observed only on the left side in M. thurstoni during courtship (Fig. 8b,c). We recorded mating scars on a female on the ventral part of the left pectoral fin at El Saltito Beach (event 209) (Fig. 5a–d), and fresh mating wounds on a female engaging in courtship train at Punta Arenas (event 210) (Fig. 5 e–h).

© Paulo Gómez-Aldana (b, c) Females M. thurstoni with fresh mating wounds indicated by white arrows (event 210 and June 2019). Photos © Maru Brito and Cecilia Mar-Ruiz (d) M. munkiana female with courtship abrasions (posterior dorsal area) and scars on her left pectoral fin resulting from the male's biting hold of her fin during copulation indicated by white arrows (May 2022). Photo © Marta D. Palacios. (e) Female M. munkiana with fresh mating wound indicated by white arrows (May 2022). Photo © Marine Bruges (f) M. munkiana females with courtship abrasions (posterior dorsal area) indicated by white arrows (May 2023). Photo © Karissa Nanetta
(a) Near-term M. mobular engaging in a courtship (event 7). Photo
We recorded mating scars and mating fresh wounds in M. munkiana on the left pectoral fins (Fig. 8d,e). We also recorded a new indicator of courtship activity for this species, the courtship abrasions, which are visible on the posterior half on M. munkiana dorsal side (Fig. 7d and Fig. 8d,f). These abrasions appear as a result of the repeated back-leaps from males onto the female’s dorsal surface during courtship, when performing the pre-copulatory behavior “piggyback leaping”. Although we did not record the number of individuals presenting these courtship abrasions due to the large size of groups and the high frequency of these abrasions, we observed the courtship abrasions on near-term pregnant females, and mature females and males. Pregnant females showing courtship abrasions on their lower dorsal area did not always have pectoral fin mating scars or wounds. These courtship abrasions were also visible on female individuals outside of the reported reproductive period (November) (Supplementary material, Fig. 2).
Breaching behavior
We only recorded breaching groups in M. munkiana, although single individuals of M. thurstoni were also frequently sighted breaching out of the water during all surveyed months in 2021–2022. During boat surveys (n = 64), M. munkiana breaching groups were recorded (n = 58 of a total 118 groups, 49%) between 2021 and 2022. We observed breaching behavior in individuals of all maturity stages (juveniles and adults) and sexes (male and female), and in pregnant females (Fig. 9a–f). During some of the breaches, the individuals shook their pectoral fins when they had a remora attached to their bodies. During courtship, breaching was not observed among individuals that were engaging in reproductive behavior, whereas breaching was observed among individuals within the larger groups who were not actively participating in the courtship. Near-term pregnant females breaching were common sights during the months of May and June (Fig. 9d–f). Different kinds of breaches were observed for this species, breaching forward to land on the ventral surface or slapping the surface of the water with their pectoral fins, breaching and landing on one side, as well as breaching to land on the dorsal surface.

© Blanca Idalia González-Garza (b) Juveniles. Photo © Juan Camilo Mora-Parra (c) non-pregnant female. Photo © Antoni Murcia (d, e) Pregnant females with fetal bulge on their dorsal side indicated by white arrows. Photos © Jay Clue (f) Pregnant female with fetal bulge and courtship abrasions on its dorsal side indicated by white arrows. Photo © Antoni Murcia
Mobula munkiana breaching behavior (a) Adult male. Photo
Discussion
Distribution and seasonality
This study extends the reproductive season described for three (M. mobular, M. thurstoni, M. munkiana) of the five mobulid species present in the Gulf of California. Previously, M. mobular and M. thurstoni were reported to reproduce during June and July, inferred from dead specimens from artisanal fisheries (Notarbartolo-di-Sciara 1988; Guerrero-Maldonado 2002; Serrano-Lopez et al. 2021). However, based on direct observations of living animals in this study, reproductive behavior occurs from May to August for M. mobular; 2 months longer than previously reported. We found that the reproductive season for M. thurstoni occurs from June to August; 1 month longer than previously reported (Serrano-Lopez et al. 2021). Mobula munkiana was observed displaying reproductive behavior from March until August; an extension of 3 months (Palacios et al. 2021). In the south-west Gulf of California, these three species are found in greatest abundance during the spring and summer (March–July) (Notarbartolo-di-Sciara G, 1988; Palacios et al. 2021; Serrano-Lopez et al. 2021), likely correlated to the abundance peak of their main prey in the region, the euphausiid Nyctiphanes simplex (Gendron 1992; Sampson et al. 2010). This time frame also coincides with the transition from the cold season (December–May), when northwest winds lead to lower sea surface temperature (SST) between 21 and 24 ºC, to the warm season (June–November), when weaker winds from the southeast bring warm tropical water from the Pacific and SST are between 27 and 31 ºC (Herguera et al. 2001; Lluch-Cota et al. 2007).
The extension of these reproductive seasons could be the result of the combination of our larger study area throughout the year than previous studies (Notarbartolo-di-Sciara G, 1988; Guerrero Maldonado 2002; Serrano-López et al. 2021; Palacios et al. 2021) and the different observational methodologies used (drone, in-water observation, citizen science, and seaplanes) which have proven to be useful in the collection of behavioral data (Fiori et al. 2017; Stewart et al. 2018; Oleksyn et al. 2021; Palacios et al. 2023). Direct observations of courtship events for M. mobular and M. thurstoni have been previously reported at offshore areas and remote archipelagos (Duffy and Tindale 2018; McCallister et al. 2020). In our study, reproductive events occurred between 4 m to 6.3 km from the Baja California Peninsula coastline or adjacent islands. The southern Gulf of California is characterized by steep slopes, with a narrow shelf and deep basins where enriched waters contribute to a high primary production close to the coast (Lavín and Marinone 2003; Lluch-Cota et al. 2007). The greatest number of reproductive events occurred at La Ventana, a channel with a maximum width of 17 km and 293 m depth between the peninsula coast and the island of Cerralvo (Nava-Sanchez et al. 1995), and at Ensenada de Muertos, where the ocean floor falls to 300 m within the first 2 km from the coast. These bathymetric features, in conjunction with high seasonal productivity during spring (Lluch-Cota et al. 2007), may favor high abundance of food and optimal conditions for the aggregation of large numbers of devil rays, facilitating the encounter of potential mating partners; similar to other reproductive aggregations in other elasmobranchs (Sims et al. 2022; Palacios et al. 2023).
Reproductive behavior
Reproductive behavior for manta ray species is well established in seven stages (Stevens et al. 2018a) of which we observed five during this study; endurance, evasion, and pre-copulation positioning were the most common.
For M. munkiana, we describe two new courtship behaviors: the courtship vortex and the piggyback leaping. Vortex formations are regularly performed by M. munkiana in the La Ventana area, probably for predator avoidance and as a coordinated feeding strategy (Palacios et al. in prep), similar to the cyclone feeding of M. alfredi in Maldives (Stevens 2016; Armstrong et al. 2021). However, we hypothesize that the courtship vortex observed in this study has a reproductive function based on the absence of feeding behavior or visible predators in the area (Higuera-Rivas et al. 2023) throughout the 5 h of the encounter. Further, the presence of sexually mature individuals of both sexes (males with elongated claspers and females with mating scars) and the observation of courtship trains entering in and out of the main formation indicates that this may represent a social and reproductive aggregation where adults assess and chose potential mates before engaging in individual courtship trains and copulation. The structure and speed of the vortex allowed physical contact among most of the individuals within the vortex, especially those situated in the center. Similar behaviors have been studied for basking sharks (Cetorhinus maximus) (Sims et al. 2022) but have never been reported for mobulid species (Yano et al. 1999; Pratt and Carrier 2001; Marshall and Bennett 2010; Deakos 2011; Stevens 2016; Stevens et al. 2018a; Mendonça et al. 2020).
In addition, a new courtship behavior was observed on M. munkiana mature males, the piggyback leaping. This courtship strategy consists of back-leaps performed by mature males onto the females back. To achieve this, males actively pursue the female, positioning themselves directly behind or beneath her to execute these leaps. This behavior occurs, while the female is right at the surface, likely to prevent the males from getting onto her dorsal surface. Since we did not observe males rubbing the backs of the females with their cephalic fins at any time (Stevens et al. 2018a), it is likely that these dorsal abrasions could be the result of the leaping on top of the female from behind as the males attempt to copulate (Fig. 7a-d). These repeated leaps create “courtship abrasions” visible several months after the end of the reproductive season (up to 3 months) for this species (Supplementary material, Fig. 2). The presence of these courtship abrasion on some adult males, were probably the result of the simultaneous back-leaps, where several males leaped onto the female at the same time. Therefore, the presence of these courtship abrasions could be used as an indicator of sexual maturity on M. munkiana.
Male biting of the pectoral fins of the female during reproductive events is a reproductive behavior in elasmobranchs (Klimley 1980; Uchida et al. 1990; Pratt and Carrier 2001; Marshall and Bennett 2010), and in mobulids is included in the pre-copulation positioning phase (Stevens et al. 2018a). This behavior enables the proper positioning of the male for the insertion of the claspers in the female cloaca, while the female remains motionless during copulation (Kajiura et al. 2000) and results in female pectoral fin abrasions, wounds, and permanent scars (Yano et al. 1999; Marshall and Bennett 2010; Stevens et al. 2018a). Devil ray species have teeth on both jaws (Notarbartolo-di-Sciara G 1987; Stevens et al. 2018b), leaving mating scars on both sides of the females’ pectoral fins when biting occurs. Wounds or mating scars (Marshall and Bennett 2010; Stevens et al. 2018a) (Fig. 8b–d) were present on the dorsal and ventral sides of M. thurstoni individual’s pectoral fins, while M. munkiana individuals also presented courtship abrasions on the posterior half of the disc.
During copulation attempts, M. munkiana males did not always wait until pre-copulation positioning (e.g., biting the female’s pectoral fin) before erecting their tail, bending their pelvic area, and making rapid pelvic thrusts for copulation (Fig. 7b,c) (Supplementary material, Video 4).
Breaching behavior
Breaching behavior, or leaping, is a commonly observed behavior in elasmobranchs, with several species (thresher shark, basking shark, white shark, eagle rays, and blacktip sharks) breaching for various hypothesized reasons including feeding, courtship, parasite removal, and predator avoidance (Curtis and Macesic 2011; Berthe et al. 2018; Gore et al. 2019). Breaching behavior for the mobulids has been hypothesized as a form of signaling mechanism to aggregate for reproduction (Marshall and Bennett 2010; Medeiros et al. 2021; Stevens et al. 2018a). The males breach to attract more potential mates and demonstrate their fitness by creating as loud a splash as possible, while the females breach to attract more potential mates from which to select a partner from during courtship (Stevens 2016). While breaching behavior may be related to reproductive behavior in M. munkiana, it is important to note that we observed all maturity stages (juveniles, adults, and pregnant females) and sexes breaching. Additionally, we found that breaching occurred year-round in the Gulf of California, including outside of the reproductive season, suggesting that it is highly likely this behavior is also driven by other biological functions as well. Based on this evidence, we hypothesize that most breaching events for M. munkiana may be a form of communication meant to attract other groups or individuals to a certain area to feed, perhaps cooperatively, or as a predator avoidance mechanism. These functions have been suggested for M. alfredi during coordinated feeding events (e.g., cyclone or chain feeding) in Maldives (Stevens 2016; Armstrong et al. 2021) and for M. birostris in estuarine environments in Brazil (Medeiros et al. 2021). Breaching behavior may also be related to parasite removal: during some of the breaching events, we observed M. munkiana actively shake pectoral fins where remoras were attached, and similar behavior has been observed for other elasmobranchs (Ritter and Brunnschweiler 2003; Brunnschweiler 2006).
Conservation implications
The southwestern Gulf of California is a reproductive area for M. mobular, M. thurstoni, and M. munkiana, based on our observations and on histological and morphological studies previously conducted in the area (Notarbartolo-di-Sciara 1988; Serrano-Lopez et al. 2021). Reproductive behavior for these three species has been observed in this area from March to August. This period extends 3 months outside of the currently established elasmobranch fishing ban in the Mexican Pacific, which extends from the first of May to the first of August. Although mobulids have been protected from target fisheries in Mexico since 2006 (NOM-029-PESC-2006), gillnets, longlines and simpleras (bottom-fixed lines with baited hooks) are used in the Gulf of California by artisanal and industrial fisheries to legally fish for stingrays and sharks during the other 9 months of the year (Bizzarro et al. 2009; Del-Valle-González-González 2018). The main threats to mobulids in the study area includes commercial fisheries, such as industrial purse seiners targeting tuna (Croll et al. 2016; Lezama-Ochoa et al. 2019), shrimp boats, artisanal gillnets, and illegal fishing activities that specifically target devil rays (Palacios unpubl data). Mobula mobular and M. thurstoni are the dominant species captured as bycatch in industrial fisheries in the region (Lezama-Ochoa et al. 2019). While the extent of the impact of bycatch and target fisheries on M. munkiana is unknown across its range (Alfaro-Cordova et al. 2017; Lezama-Ochoa et al. 2019), high rates of mobulid bycatch in artisanal gillnets have been observed within the study area (Del-Valle-González-González 2018; Palacios unpubl data). The lack of spatial management for shark and ray fisheries (Bizzarro et al. 2007) and the use of non-selective gear (gillnets) in Mexico, coupled with the limited enforcement of existing regulations potentially puts critical habitats at risk, even for protected species like mobulids (Salomón-Aguilar 2015; Jabado et al. 2023). Establishing greater spatial and temporal restrictions on the use of gillnets along the coast and islands in the southwestern Gulf of California, especially around critical habitats, such as reproductive grounds (La Ventana and Ensenada de Muertos area) and nursery areas (Espiritu Santo Archipelago) (Palacios et al. 2021), may help reduce the impact of bycatch of these species during key life periods.
The results of this study indicate reproductive areas are within 6.3 km of the coast, where we observed surface aggregations of the three devil ray species. This distribution makes them vulnerable not only to bycatch, but to other anthropogenic threats such as an increasing coastal development in the region and the associated sound and chemical pollution, habitat loss, and boat traffic. During our surveys, we observed reproductive behavior at the surface, creating the potential for individuals engaging in reproduction to be exposed to boat strikes potentially resulting in lethal or sublethal injuries (Womersley et al. 2022; Strike et al. 2022). Furthermore, currently unregulated tourist activities offering free diving and snorkeling with devil rays, especially M. munkiana, are growing in the area with more than 80 tourism companies (Bruges et al. in prep) providing new economic opportunities to local communities. Currently, little is known about the extent to which these activities affect the reproductive behavior and movement patterns of these species. However, unregulated tourism has negatively impacted manta rays in the Maldives, Australia (Venables 2013; Venables et al. 2016) and Mexico (Gómez-García et al. 2021), showing to disrupt or stop natural behaviors during 37% of the observations (Murray et al. 2020). The establishment of management measures, including studies of the optimal carrying capacity for mobulid tourism (Zekan et al. 2022), and codes of conduct to observe and interact with these species may help mitigate the negative impacts of tourism activities (Murray et al. 2020) at reproductive grounds while offering economic benefits to local communities (O’Malley et al. 2013).
Only four of the 221 reproductive behavior events occurred inside a marine protected area (MPA) (Espiritu Santo Archipelago and Cabo Pulmo), with only Cabo Pulmo being a strict no-take MPA. This is of potential concern, because near-term mobulids are routinely thought to mate within hours or days of giving birth (Stevens et al. 2018a). This is supported by our observations of heavily pregnant M. mobular and M. munkiana females engaging in courtship behavior indicating it is likely that birthing areas are located adjacent to these nursery areas, as previously described for M. munkiana (Palacios et al. 2021). This suggests that further spatial protection of reproductive areas could be useful to simultaneously protect both nursery, courtship, and mating areas. Recently, several Important Shark and Ray Areas (ISRA) (Hyde et al. 2022) have been established in the Mexican Pacific and Gulf of California (Jabado et al. 2023); however, some of the critical habitats for reproductive behavior reported in this study such as the Ensenada de Muertos area are not covered inside the designated polygons of ISRA. The existing MPAs in the region prove inadequate for mobulids, needing immediate action to improve spatial protection against gillnets, industrial fisheries, and other anthropogenic threats.
Data availability
The dataset of reproductive event observations used during the current study is available from the corresponding author on reasonable request.
References
Alfaro-Cordova E, Del-Solar A, Alfaro-Shigueto J, Mangel JC, Diaz B, Carrillo O et al (2017) Captures of manta and devil rays by small–scale gillnet fisheries in northern Peru. Fish Res 195:28–36. https://doi.org/10.1016/j.fishres.2017.06.012
Armstrong AO, Stevens GMW, Townsend KA, Murray A, Bennett MB, Armstrong AJ et al (2021) Reef manta rays forage on tidally driven, high density zooplankton patches in Hanifaru Bay. Maldives Peerj 9:e11992. https://doi.org/10.7717/peerj.11992
Arnés-Urgellés C, Hoyos-Padilla EM, Pochet F, Salinas–de–León P, (2018) First observation on the mating behaviour of the marbled ray, Taeniurops meyeni, in the tropical Eastern Pacific. Env Biol Fish 101:1693–1699. https://doi.org/10.1007/s10641-018-0818-z
Berthe C, Waqalevu VP, Latry L, Besson M, Lerouvreur F, Siu G et al (2018) Distribution patterns of ocellated eagle rays, Aetobatus ocellatus, along two sites in Moorea Island, French Polynesia. Cybium 42(4) 313–320 https://doi.org/10.26028/cybium/2018-424-002
Bizzarro JJ, Smith WD, Hueter RE, Tyminski J, Márquez–Farías JF, Castillo–Géniz JL et al (2007) The status of shark and ray fishery resources in the Gulf of California: applied research to improve management and conservation. Moss Landing Marine Laboratories Tech Pub 2009–01
Bizzarro JJ, Smith WD, Hueter RE, and Villavicencio–Garayzar CJ (2009) Activities and Catch Composition of Artisanal Elasmobranch Fishing Sites on the Eastern Coast of Baja California Sur, Mexico. Bull South Calif Acad Sci Vol. 108: Iss. 3
Broadhurst MK, Laglbauer BJL, Bennett MB (2019) Gestation and size at parturition for Mobula kuhlii cf. eregoodootenkee. Environ Biol Fish 102:1009–1014. https://doi.org/10.1007/s10641-019-00886-3
Brunnschweiler JM (2006) Shark sucker–shark interaction in two carcharhinid species. Mar Ecol 27:89–94. https://doi.org/10.1111/j.1439-0485.2005.00052.x
Brusca RC, Findley LT, Hastings PA, Hendrickx ME, Cosio JT, van-der-Heiden AM, (2005) Macrofaunal diversity in the Gulf of California. In: Carton EJL, Ceballos G, Felger SR (eds) Biodiversity, ecosystems, and conservation in Northern Mexico. Oxford University Press, New York, pp 179–202
Carpenter M, Griffiths C (2023) ‘Flash Mobula’: first observations of courtship behaviour of the shortfin devil ray Mobula kuhlii. Afr J Mar Sci 45(1):51–56. https://doi.org/10.2989/1814232X.2022.2158131
Childs JN (2001) The occurrence,abitat use and behavior of sharks and rays associating with topographic highs in the northwestern Gulf of Mexico. Master’s Dissertation, Texas A&M University
Coles RJ (1910) Observations on the habits and distribution of certain fishes taken on the coast of North Carolina. Bulletin of the AMNH, v28, article 28.
Conrath CL and Musick JA (2012) Reproductive biology of elasmobranchs. In: JC Carrier JA Musick, and MR Heithaus (eds), Biology of sharks and their relatives CRC Press, Florida, pp 291–312
Couturier LIE, Marshall AD, Jaine FRA, Kashiwagi T, Pierce SJ, Townsend KA et al (2012) Biology, ecology and conservation of the mobulidae. J Fish Biol 80:1075–1119. https://doi.org/10.1111/j.1095-8649.2012.03264.x
Croll DA, Newton KM, Weng K, Galván-Magaña F, O’Sullivan J, Dewar H (2012) Movement and habitat use by the spine–tail devil ray in the Eastern Pacific Ocean. Mar Ecol Prog Series 465:193–200. https://doi.org/10.3354/meps09900
Croll DA, Dewar H, Dulvy NK, Fernando D, Francis MP, Galván-Magaña F et al (2016) Vulnerabilities and fisheries impacts: the uncertain future of manta and devil rays. Aqua Conserv Mar Freshw Ecosyst 26:562–575. https://doi.org/10.1002/aqc.2591
Curtis TH, Macesic LJ (2011) Observations of breaching behaviour in juvenile bull sharks, Carcharhinus leucas. Florida Scientist 74:253–257
Deakos MH (2011) The reproductive ecology of resident manta rays Manta alfredi off Maui, Hawaii, with an emphasis on body size. Env Biol Fish 94:1–14. https://doi.org/10.1007/s10641-011-9953-5
Del–Valle–González–González (2018) Diversidad de los peces batoideos de la zona sur de la Isla Espíritu Santo, BCS, México. Master’s Dissertation, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas.
Duffy CA, Tindale SC (2018) First observation of the courtship behaviour of the giant devil ray Mobula mobular (Myliobatiformes: Mobulidae). NZ J Zool 45:387–394. https://doi.org/10.1080/03014223.2017.1410850
Dulvy NK, Pardo SA, Simpfendorfer CA, Carlson JK (2014) Diagnosing the dangerous demography of manta rays using life history theory. PeerJ 2:e400. https://doi.org/10.7717/peerj.400
Ehemann N, Acosta-Rodríguez E, Tagliafico A, Pelletier N, Stevens G (2022) Manta and devil ray species occurrence and distribution in Venezuela, assessed through fishery landings and citizen science data. J Fish Biol. https://doi.org/10.1111/jfb.15088
Fiori L, Doshi A, Martinez E, Orams MB, Bollard-Breen B (2017) The use of unmanned aerial systems in marine mammal research. Remote Sens 9(6):543. https://doi.org/10.3390/rs9060543
Gendron D (1992) Population structure of surface of daytime surface swarms of Nyctiphanes simplex (Crustacea, Euphausiacea) in the Gulf of California, México. Mar Ecol Prog Ser 87:1–6. https://doi.org/10.3354/meps087001
Germanov ES, Bejder L, Chabanne DBH, Dharmadi D, Hendrawan IG, Marshall AD et al (2019) Contrasting habitat use and population dynamics of reef manta rays within the nusa penida marine protected area. Indonesia Front Mar Sci. https://doi.org/10.3389/fmars.2019.00215
Gómez-García MJ, Blázquez-Moreno MC, Stewart JD, Leos-Barajas V, Fonseca-Ponce IA, Zavala-Jiménez AA et al (2021) Quantifying the effects of diver interactions on Manta Ray behavior at their aggregation sites. Front Mar Sci 8:639772. https://doi.org/10.3389/fmars.2021.639772
Gore M, Abels L, Wasik S, Saddler L, Ormond R (2019) Are close–following and breaching behaviours by basking sharks at aggregation sites related to courtship? J Mar Biol Assoc UK 99(3):681–693. https://doi.org/10.1017/S0025315418000383
Guerrero Maldonado LA (2002) Captura comercial de elasmobranquios en la costa suroccidental del Golfo de California, México. Bachelor Dissertation, Universidad Autónoma De Baja California Sur
Herguera JC, Ripa P, Bernal G (2001) Variabilidad oceanográfica y climática en el Bajo Golfo de California: Influencias del Trópico y Pacífico Norte. Cienc Mar 27(4):595–617
Higuera-Rivas JE, Hoyos-Padilla EM, Elorriaga-Verplancken FR, Rosales-Nanduca H, Rosenthal R, Urbán RJ (2023) Orcas (Orcinus orca) use different strategies to prey on rays in the Gulf of California. Aquat Mamm 49(1):7–18. https://doi.org/10.1578/AM.49.1.2023.7
Hyde CA, Notarbartolo–di–Sciara G, Sorrentino L, Boyd C, Finucci, B, Fowler, et al (2022) Putting sharks on the map: A global standard for improving shark area–based conservation. Front Mar Sci 9:968853. https://doi.org/10.3389/fmars.2022.968853
IUCN (2020) The IUCN red list of threatened species. version 2020–2. Available at: https://www.iucnredlist.org/ (Accessed 20 May 2023).
Jabado RW, García–Rodríguez E, Kyne PM, Charles R, Gonzalez Pestana A, Priest MA, Battle–Morera A, Notarbartolo–di–Sciara G (2023) Central and South American Pacific: A regional compendium of Important Shark and Ray Areas. Dubai: IUCN SSC Shark Specialist Group
Kajiura SM, Sebastian AP, Tricas TC (2000) Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environ Biol Fish 58:23–31. https://doi.org/10.1023/A:1007667108362
Klimley AP (1980) Observations of courtship and copulation in the nurse shark, Ginqlymostoma cirratum. Copeia 1980:878–882
Lavin MF and Marinone SG (2003) An overview of the physical oceanography of the Gulf of California. In: Velasco–Fuentes U, Sheinbaum J and Ochoa de la Torre JL (eds) Nonlinear processes in geophysical fluid dynamics. O. Kluwer Academic, Holland pp 173–204.
Lezama-Ochoa N, Hall M, Román M, Vogel N (2019) Spatial and temporal distribution of mobulid ray species in the eastern Pacific Ocean ascertained from observer data from the tropical tuna purse–seine fishery. Environ Biol Fish 102:1–17. https://doi.org/10.1007/s10641-018-0832-1
Lluch-Cota SE, Aragon-Noriega EA, Arreguín-Sánchez F, Aurioles-Gamboa D, Bautista-Romero JJ, Brusca RC, Sierra-Beltrán AP (2007) The Gulf of California: review of ecosystem status and sustainability challenges. Prog Oceanogr 73(1):1–26. https://doi.org/10.1016/j.pocean.2007.01.013
Manual EH (2004) Captive Care of Sharks, Rays, and their Relatives/A Special Publication of the Ohio Biological Survey. Inc.–Columbus, Ohio, 586
Marshall AD, Bennett MB (2010) Reproductive ecology of the reef manta ray Manta alfredi in southern Mozambique. J Fish Biol 77:169–190. https://doi.org/10.1111/j.1095-8649.2010.02669.x
McCallister M, Mandelman J, Bonfil R, Danylchuk A, Sales M, Ajemian M (2020) First observation of mating behavior in three species of pelagic myliobatiform rays in the wild. Enviro Biol Fish 103:163–173. https://doi.org/10.1007/s10641-019-00943-x
Medeiros AM, Ari C, Monteiro-Filho ELA (2021) Environmental factors involved in breaching behavior of manta rays in estuarine waters. Mar Ecol Prog Ser 674:203–219. https://doi.org/10.3354/meps13815
Mendonça SA, Macena BCL, Araújo CBB, Bezerra NPA, Hazin FHV (2020) Dancing with the devil: courtship behaviour, mating evidence and population structure of the Mobula tarapacana (Myliobatiformes: Mobulidae) in a remote archipelago in the equatorial mid–Atlantic Ocean. Neotrop Ichthyol 18(3):e200008. https://doi.org/10.1590/1982-0224-2020-0008
Murray A, Garrud E, Ender I, Lee-Brooks K, Atkins R, Lynam R et al (2020) Protecting the million–dollar mantas; creating an evidence–based code of conduct for manta ray tourism interactions. J Ecot 19:132–147. https://doi.org/10.1080/14724049.2019.1659802
Nava-Sanchez E, Cruz-Orozco R, Gorsline DS (1995) Morphology and sedimentology of two contemporary fan deltas on the southeastern Baja California Peninsula. Mexico Sediment Geol 98(1–4):45–61. https://doi.org/10.1016/0037-0738(95)00026-5
Notarbartolo-di-Sciara G (1987) A revisionary study of the genus Mobula Rafinesque, 1810 (Chondrichthyes: Mobulidae) with the description of a new species. Zool J Linn Soc 91:1–91. https://doi.org/10.1111/j.1096-3642.1987.tb01723.x
Notarbartolo-di-Sciara G, Adnet S, Bennett M, Broadhurst MK, Fernando D, Jabado RW et al (2019) Taxonomic status, biological notes, and conservation of the longhorned pygmy devil ray Mobula eregoodoo (Cantor 1849). Aquat Conserv Mar Freshw Ecosyst 30:104–122. https://doi.org/10.1002/aqc.3230
Notarbartolo–di–Sciara G, (1988) Natural history of the rays of the genus mobula in the gulf of California. Fish Bul 86:45–66
O’Malley MP, Lee-Brooks K, Medd HB (2013) The global economic impact of manta ray watching tourism. PLoS ONE 8:e65051. https://doi.org/10.1371/journal.pone.0065051
Oleksyn S, Tosetto L, Raoult V, Joyce KE, Williamson JE (2021) Going batty: the challenges and opportunities of using drones to monitor the behaviour and habitat use of rays. Drones 5(1):12. https://doi.org/10.3390/drones5010012
Palacios MD, Hoyos-Padilla EM, Trejo-Ramı́rezCroll ADA, Galván-Magaña F, Zilliacus KM et al (2021) Description of first nursery area for a pygmy devil ray species (Mobula munkiana) in the Gulf of California, Mexico. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-020-80506-8
Palacios MD, Stewart JD, Croll DA, Cronin MR, Trejo-Ramírez A, Stevens GM et al (2023) Manta and devil ray aggregations: conservation challenges and developments in the field. Front Mar Sci 10:1148234. https://doi.org/10.3389/fmars.2023.1148234
Pratt HL, Carrier JC (2001) A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Enviro Biol Fish 60:157–188. https://doi.org/10.1023/A:1007656126281
Rambahiniarison J, Agustines A, Alexopoulos K, Araujo G, Armstrong AO, Arnold S et al (2022) Distribution of the reef manta ray Mobula alfredi and the oceanic manta ray Mobula birostris in the Philippines: a collaborative effort for conservation. J Fish Biol. https://doi.org/10.1111/jfb.15283
Rangel BS, Afonso AS, Garla R (2022) Female wound records suggest mating periods for the Caribbean reef shark at an insular marine protected area from the Equatorial Atlantic Ocean. J Fish Biol 101(6):1591–1594. https://doi.org/10.1111/jfb.15212
Ritter EK, Brunnschweiler JM (2003) Do sharksuckers, Echeneis naucrates, induce jump behaviour in blacktip sharks, Carcharhinus limbatus? Mar Freshwater Behav Physiol 36:111–113. https://doi.org/10.1080/1023624031000119584
Salomón-Aguilar CA (2015) Zonas prioritarias de conservación de rayas y mantarrayas en el noroeste del Pacífico mexicano. Ciencia Pesquera 23(2):77–99
Sampson L, Galván-Magaña F, De Silva-Dávila R, Aguíñiga-García S, O’Sullivan J (2010) Diet and trophic position of the devil rays Mobula thurstoni and Mobula japanica as inferred from stable isotope analysis. J Mar Biol Assoc UK 90(5):969–976. https://doi.org/10.1017/S0025315410000548
Santamaria-Del-Angel E, Alvarez-Borrego S, Millán-Nuñez R, Muller-Karger FE (1999) Sobre el efecto de las surgencias de verano en la biomasa fitoplanctónica del Golfo de California. Rev Soc Mex Hist Nat 49:207–212
Serrano-López JN, Soto-López K, Ochoa-Báez RI, O’Sullivan J, Galván-Magaña F (2021) Morphometry and histology to assess the maturity stage of three endangered devil ray species (Elasmobranchii: Mobulidae) from the Gulf of California. Aquat Conserv Mar Freshwater Ecosyst 31(7):1624–1635. https://doi.org/10.1002/aqc.3548
Setyawan E, Erdmann MV, Lewis SA, Mambrasar R, Hasan AW, Templeton S et al (2020) Natural history of manta rays in the Bird’s Head Seascape, Indonesia, with an analysis of the demography and spatial ecology of Mobula alfredi (Elasmobranchii: Mobulidae). J Ocean Sci Found 36:49–83. https://doi.org/10.5281/zenodo.4396260
Setyawan E, Stevenson BC, Izuan M, Constantine R, Erdmann MV (2022) How big is that manta ray? a novel and non-invasive method for measuring reef manta rays using small drones. Drones 6(3):63. https://doi.org/10.3390/drones6030063
Sims DW, Berrow SD, O’Sullivan KM, Pfeiffer NJ, Collins R, Smith KL et al (2022) Circles in the sea: annual courtship “torus” behaviour of basking sharks Cetorhinus maximus identified in the eastern North Atlantic Ocean. J Fish Biol 101(5):1160–1181. https://doi.org/10.1111/jfb.15187
Smith M, Warmolts D, Thoney D, Hueter R (2004) The elasmobranch husbandry manual: captive care of sharks, rays and their relatives. Special Publication of the Ohio Biological Survey, Columbus, Ohio
Sobral AFL (2013) Biology, ecology and conservation of Mobulid rays in the Azores. Master’s Dissertation, Universidade dos Acores
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57:476–494. https://doi.org/10.1006/jmsc.2000.0724
Stevens GMW, Hawkins JP, Roberts CM (2018a) Courtship and mating behavior of manta rays Mobula alfredi and M. birostris in the Maldives. J Fish Biol 93:344–359. https://doi.org/10.1111/jfb.13768
Stevens GMW, Fernando D, Dando M and Notarbartolo–di–Sciara G (2018b) Guide to the Manta and Devil Rays of the World In: Wild Nature Press, United Kindom
Stevens GMW (2016) Conservation and population ecology of manta rays in the Maldives. PhD Dissertation, University of York.
Stewart JD, Jaine FR, Armstrong AJ, Armstrong AO, Bennett MB, Burgess KB et al (2018) Research priorities to support effective manta and devil ray conservation. Front Mar Sci 5:314. https://doi.org/10.3389/fmars.2018.00314
Strike EM, Harris JL, Ballard KL, Hawkins JP, Crockett J, Stevens GMW (2022) Sublethal injuries and physical abnormalities in Maldives Manta Rays. Front Mar Sci, Mobula alfredi and Mobula birostris. https://doi.org/10.3389/fmars.2022.773897
Uchida S, Toda M and Kamei Y (1990) Reproduction of elasmobranchs in captivity. In: Pratt HL, Gruber Jr SH and Taniuchi T (ed.) Elasmobranchs as Living Resources: Advances in Biology, Ecology, Systematics and Status of the Fisheries, NOAA Tech Rep NMFS 90, pp 211–237
Venables S, McGregor F, Brain L, Mvan-Keulen (2016) Manta ray tourism management, precautionary strategies for a growing industry: a case study from the Ningaloo Marine Park, Western Australia. Pac Conserv Biol 22:295–300. https://doi.org/10.1071/PC16003
Venables S (2013) Short term behavioral responses of manta rays, Manta alfredi, to tourism interactions in Coral Bay, Western Australia. PhD Dissertation, Murdoch University
Whitehead DA, Gayford JH, Hoyos EM et al (2022) First description of a sex segregated aggregation of silky sharks (Carcharhinus falciformis) and the frequency and distribution of mating wounds off the tip of the Baja California Peninsula. Environ Biol Fish 105:953–960. https://doi.org/10.1007/s10641-022-01297-7
Whitney NM, Pratt HL Jr, Carrier JC (2004) Group courtship, mating behaviour and siphon sac function in the whitetip reef shark. Triaenodon Obesus Anim Behav 68(6):1435–1442. https://doi.org/10.1016/j.anbehav.2004.02.018
Whitney NM, Pratt HL Jr, Pratt TC, Carrier JC (2010) Identifying shark mating behaviour using three–dimensional acceleration loggers. Endang Species Res 10:71–82. https://doi.org/10.3354/esr00247
Womersley FC, Humphries NE, Queiroz N, Vedor M, da–Costa I, Furtado M, et al (2022) Global collision–risk hotspots of marine traffic and the world’s largest fish, the whale shark. Proc Natl Acad Sci 119:e2117440119. https://doi.org/10.1073/pnas.2117440
Yano K, Sato F, Takahashi T (1999) Observations of mating behavior of the manta ray, Manta birostris, at the ogasawara islands, Japan. Ichthyol Res 46:289–296. https://doi.org/10.1007/BF02678515
Zekan B, Weismayer C, Gunter U, Schuh B, Sedlacek S (2022) Regional sustainability and tourism carrying capacities. J Cleaner Produ 339:130624. https://doi.org/10.1016/j.jclepro.2022.130624
Acknowledgements
The authors are thankful to the two reviewers for their constructive feedback that greatly contributed to enhancing the quality of our manuscript. We are thankful to captains Luciano and Juan Lucero, Armando, Juan and Felipe Cuevas, all Mobula Conservation students and volunteers, and to Ben Fiscella Meissner for their help and field assistance. The authors are grateful to Paulo Gómez Aldana, Adriá Bosch-Soler, Maru Brito, Afelandra González-Cibrián, Shawn Hendrich, Jay Clue, Cecilia Mar-Ruiz, Karissa Nanetta, Blanca Idalia González-Garza, Juan Camilo Mora-Parra, Marine Bruges, Laurent Ballesta, Wojciech Dopierala, Sara Jaramillo, and Antoni Murcia for their observations, photographs and videos. The authors are grateful to Dive Ninja Expeditions guides (Donna, Ellen, Marco, Juan Camilo, and others) for collecting mobula data and helping with field access and to Ocean Life Flights and Baja Expeditions for their observations and aerial support. The authors are grateful to Dive Gurus and Luke Inman for their support with 2017 observations. We are grateful to Walter Munk Foundation for the Oceans for their support and assistance throughout 2021–2022. The authors are grateful to Manta Trust and Fundación México Azul for their support. The authors are grateful to Laurent Ballesta and his Gombessa Expeditions team for their assistance in the collection of data during 2022. F.G.M and R.G.A thanks to Instituto Politécnico Nacional for fellowships (COFAA and EDI). Additional thanks to the Comisión Nacional de Áreas Naturales Protegidas (CONANP)—Islas del Golfo/Parque Nacional Zona Marina del Archipiélago Espíritu Santo, for field work authorization and support.
Funding
The research leading to these results received funding from the Walter Munk Foundation for the Oceans, Monterey Bay Aquarium Foundation, The Explorers Club (2022 Exploration Fund Grant), Dive Ninja Expeditions, Blancpain Manufacture of Fine Watchmaking, and the Blancpain Ocean Commitment. The author M.D.P was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT), BEIFI, and Walter Munk Foundation for the Oceans.
Author information
Authors and Affiliations
Contributions
MDP, JDS, MRC, NLO, KMZ, RGA, FGM, and DAC conceived the paper. MDP, SVH, and SAKHM conducted the fieldwork. MDP wrote the main manuscript text. MDP and ATR designed and elaborated the figures. All authors contributed to writing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Research was conducted under the research permit PPF/DGOPA-024/20 issued by Comisión Nacional de Acuacultura y Pesca with authorization of Comisión Nacional de Áreas Naturales Protegidas. No specimens were collected during this research. The drone used in this study is registered at the AFAC under the folder MX-F-2-2303-036.
Additional information
Responsible Editor: J. Carlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file4(MP4 349426 KB)
Supplementary file5 (MP4 56031 KB)
Supplementary file6 (MP4 181163 KB)
Supplementary file7 (MP4 331913 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palacios, M.D., Trejo-Ramírez, A., Velázquez-Hernández, S. et al. Reproductive behavior, seasonality, and distribution of three devil ray species (Mobula mobular, M. thurstoni, and M. munkiana) in the Southern Gulf of California, Mexico. Mar Biol 171, 12 (2024). https://doi.org/10.1007/s00227-023-04314-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04314-0