Methods to obtain drought resistant plants
Field of the invention.
The present invention relates to genetic engineering, specifically to transgenesis and cisgenesis processes of plants and tissues of vegetal origin, and more particularly to methods for selecting genetically transformed plant cells avoiding the use of resistance genes toxic substances as selectable markers, and without the transformed cell to produce a protein and/or a new or different metabolite with a potential negative effect on the plant cell, the plant or its end users and intermediaries. Thus, the method of the present invention uses a nucleotide sequences comprising a nucleic acid sequence that promotes the inhibition of endogenous trehalase enzyme, which confers the plants derived from this method of selection drought tolerance.
Background of the invention.
As it is known, when genetic material is introduced into a cell population by transformation only a certain number of cells will be transformed successfully. After transformation, the transformed cells must be identified and selected from a population of transformed and untransformed cells. The identification and separation of transformed cells have been carried out using traditional methods of "negative selection," where the transformed cells can survive and grow, while untransformed cells are subject to growth inhibition or their elimination by a substance, which by virtue of their transformation the transformed cells can tolerate. For this purpose, the cell usually introduces a selection gene aside from the transgene of interest.
This selection gene typically provides an antibiotic or herbicide resistance with which the genetically transformed cells can be identified. After transformation, the transformed and untransformed cell population is then grown in a culture medium containing the antibiotic or herbicide to which the transformed cells are resistant by virtue of the selection gene, thus allowing the transformed cells that do not contain the antibiotic or herbicide resistant gene to be subject to growth inhibition, so that only the transformed cells are able to survive and grow because of the presence of the introduced gene selection.
Among others, these examples include patents US6174724 and EP0131623 that use as a selection marker the gene encoding the enzyme neomycin phosphotransferase type II {nptll), which confers resistance to certain antibiotics of the family of aminoglycosides (kanamycin, neomycin, G418, paromycin), and the patent US4727027 using the hygromycin phosphotransferase gene (hpt) to give resistance to the hygromycin antibiotic.
Despite its observed effectiveness, negative selection methods have some disadvantages. For example, untransformed cells die because of the presence of antibiotics or herbicides in the growth medium, and hence there is the risk that not only untransformed but also transformed cells may die, because the damaged untransformed cells or in the process of elimination can excrete toxic compounds.
Another very important disadvantage is that generally the use of such selection genes providing resistance to a toxic compound is not recommended from the standpoint of environmental and food security for genetically modified and cisgenic crops, which are introduced and released at large-scale in the environment, particularly food crops.
A further disadvantage of negative selection is that cells or plant tissues treated with toxic substances become more susceptible to bacterial infection. This presents a problem when using Agrobacterium as a transformation vector, because the tissues or cells may sometimes show an overgrowth of the bacteria although antibiotics are used to prevent it.
Among the positive selection methods, we can mention patents US6444878 and US5767378 that use genes for metabolism of compounds that are toxic to the cell. The first patent describes the use of a nucleotide sequence which encodes for the glucosamine-6-phosphate deaminase gene as a resistant gene of genetically transformed cells grown in a culture medium containing the toxic metabolite glucosamine or a derivative thereof. The second patent describes the use of a group of genes that encode phosphomanoisomerases, phosphomanomutases mannose epimerases, etc. involved in the metabolism of mannose or its derivatives or precursors, and the addition of any of these toxic compounds in the culture means of selection. Both cases and the use of antibiotic and herbicide resistant genes can cause the genetically transformed cell to produce a foreign protein that besides of resulting an allergen involves a metabolic cost to the transformed organism that has already been selected and does not require this product.
Recently, patent US7449290 describes a selection method of genetically transformed cells using as selection marker a nucleotide sequence that encodes a protein having trehalase activity, which catalyzes the trehalose sugar or its methylated or halogenated derivatives. In this method, the trehalose enzyme is over expressed to allow the genetically transformed cell to survive toxic concentrations of the metabolite added to the selection medium. As in previous cases, the genetically modified organism presents an overproduction of a protein that is unnecessary after the selection process, and involves the addition of an extra compound to the culture medium of selection.
The disadvantages mentioned above can be overcome, at least substantially, by a positive selection with the method of the present invention that allows the identification and isolation of genetically transformed cells without eliminating by intoxication the untransformed cells of the population and without the co-introduction of antibiotic resistant genes, herbicides and other compounds, also avoiding the need to add an extra compound to the culture medium. The method according to the present invention also avoids the extra production of a protein or polypeptide that is unnecessary at the end of the selection process.
Patents US7214858 and US7560613 describe nucleic acid fragments encoding enzymes involved in trehalose metabolism in plants and seeds to obtain genetically modified plants. They propose the use of gene sequences such as trehalase, trehalose phosphate synthase and trehalose 6-phosphate phosphatase. These documents only present experimental evidence in
transgenic plants expressing trehalose 6-phosphate phosphatase, without offering experimental evidence for the gene encoding trehalase. However, Perry and col. performed sequence alignments analysis of the trehalase enzyme of Glycine max, Neurospora crassa, maize and soybeans. It is from these computational studies that they propose the use of a trehalase gene to produce genetically modified plants where the trehalose metabolism may be altered in plants, but without suggesting its use as a screening method.
Therefore, it is necessary to provide efficient methods to obtain genetically transformed cells without the introduction of foreign genes, by that avoiding unnecessary production of proteins and/or peptides unrelated to these cells, and which are unnecessary.
Brief description of the figures.
Figure 1. Shows a map of the gene expression unit for transforming maize.
Figure 2. Shows the strategy for generating transgenic maize plants tolerant to PEG 8000 by using embryogenic callus and biolistic.
Figure 3. Shows the selection of seeds tolerant to PEG 8000 as a simulator drought test at
T1 and verification of molecular insertion of the functional unit corresponding to 35S NPTII. Lanes 1 and 2 correspond to papaya plants transformed with the same vector, where the expected signal 1 .8 Kb is seen; lane 3 negative control of maize; lane 4 maize line T4; lane 5 line T7; lane 6 line T12; lane 7 line T1 ; lane 8 line 6; lane 9 DNA of undigested T4 line.
Figure 4. Shows the cisgen and a segment of the promoter amplification using specific oligonucleotides from the total DNA of plants Bt7 GM and control. Shows a representative amplification; however, all seeds that germinated under stress gave a positive PCR signal.
Figure 5. Shows the RT-PCR quantification by real-time accumulation of mRNA of the gene encoding WT trehalase, wild; T1 and T4; GMs; H20, irrigated; SEQ, drought.
Figure 6. Shows seedlings grown in pots and tubes to observe a possible effect of the container.
Figure 7. Shows plants after two months of germination in PVC tubes designed to apply maize drought and allow physiological and soil measurements.
Figure 8. Shows growth differences between plant varieties. B73 GM plants have a larger size when compared with B73 control plant (left panel). Shows a size comparison; the leave may be undulating or exuberant, thus maintaining higher photosynthetic surface (right panel).
Figure 9. Shows male flowering in the B73 T1 and T4 GM line.
Figure 10. Shows the appearance of the ear of maize in both GM plants, each plant having
3 to 5 ears. The phenotype is substantially identical to control plants.
Figure 11. Shows the growth measurement in size from seed in cylinder to senescence.
Indicates the start of the drought, which lasted two weeks for GM lines. After a week the Creole maizes had to be watered because of the symptoms developed in this application of severe drought.
Figure 12. Shows physiological comparison between plant varieties.
Figure 13. Shows the phenotype of plants under drought treatment. Each panel shows to the left the plant subject to drought, while to the right is the control plant grown under irrigation. The panels above show the Creole maizes, where drought plants lost turgor and size. The panels below show in the middle the control plant with a significant difference in size. Plant GM T1 (far left) should be noted, which did not show a statistically significant reduction compared to its control under irrigation.
Figure 14. Shows a photosynthesis measurement comparison in wet and dry conditions in
B73. Time measurements: Day 1 : normal irrigation. Day 6: five days after drought onset. Day 13: thirteen days after drought onset, and the drought is suspended. Day 22: Ten days after the suspension of drought.
Figure 15. Shows a measurement comparison of water conductance in wet and drought conditions in B73.
Figure 16. Shows a measurement comparison of water content in wet and drought conditions in B73.
Figure 17. Shows a measurement comparison of relative humidity percentage under wet and drought conditions in B73, in both plant and soil.
Figure 18. Shows a comparison measurement of C02 content in wet and drought conditions in B73.
Figure 19. Shows a measurement comparison of transpiration in wet and drought conditions in B73.
Figure 20. Shows the HPLC spectra of trehalose peaks obtained in the samples analyzed:
(A) B73 control-1 , (B) B73 control-2, (C) B73 control-3, (D) B73 T1 -1 , (E) B73 T1-2, (F) B73 T1 -3, (G) B73 T4-1 , (H) B73 T4-2 and (I) B73 T4.
Figure 21. Shows a chart of trehalose percentages present in the plants analyzed by HPLC with an increase in genetically modified plants according to the invention with respect to what was detected in samples from control B73.
Figure 22. Shows the levels of glucose production from hydrolysis of trehalose in an incubation time of three hours.
Figure 23. Shows the electrophoretic profile of LD-PCR (1 % agarose; 80 volts; 7 μί of sample) amplified without markup (without a32P-dCTP) in order to know if the amplification from the cDNA was achieved with what we had. NTC Negative control (no DNA); 2: B73 T4 in humidity.
Figure 24. Shows the signals obtained from hybridization of nylon membranes contained plasmids with radioactively labeled probes of B73 control and genetically modified plants (T1 and T4). (1 ) B73 control in humidity, (2) B73 control in drought, (3) B73 T1 in drought, (4) B73 T1 in humidity, (5) B74 T4 in humidity; and (6) B73 T4 in drought. The letters A, B, C, D and E show the columns with duplicates, whereas numbers 1 to 12 show the lines or horizontal lines where the genes induced by drought were placed by duplicated.
Figure 25. Shows a graph of induction or repression behavior of genes involved in abiotic stress response detected in control and genetically modified B73 plants according to the invention.
Figure 26. Shows a graph of the induction or repression behavior of genes involved in the photosynthesis detected in control and genetically modified B73 plants according to the invention,
Figure 27. Shows a graph of the induction or repression behavior of genes involved in biotic stress responses detected in control and genetically modified B73 plants according to the invention.
Figure 28. Shows a graph of the induction or repression behavior of genes associated with carbohydrate metabolism detected in control and genetically modified plants B73 according to the invention.
Figure 29. Shows a graph of induction or repression behavior of genes classified as unknown and/or hypothetical proteins detected in control and genetically modified B73 plants according to the invention.
Detailed description of the invention.
The present invention describes a method for selecting genetically transformed cells in which a nucleotide sequence of interest has been incorporated that confers the transformed cells a selective advantage. The selective advantage possessed by the transformed cells may be caused to their best ability to untransformed cells to survive and grow in an environment with low water availability to cells.
One objective of the present invention is to provide an efficient method of selection of transformants, for example, plants that remove the disadvantages mentioned above for methods using selectable marker genes. To achieve this objective the present invention provides a method for identifying and/or selecting cells that have a metabolic advantage as a result of being transformed from a population of transformed and untransformed cells, which are grown on or in a culture medium containing an osmo-regulating agent such as polyethylene glycol 8000 (PEG800), mannitol, sorbitol, NaCI or any other osmo-regulating agent, wherein the method comprises:
a) Introducing in a cell at least one nucleotide sequence of interest and at least one nucleotide sequence of selection to obtain a genetically transformed cell, where the nucleotide sequence of selection comprises a region encoding an endogenous inhibitor of trehalase;
b) Placing the population of transformed and untransformed cells in contact with a culture medium comprising a concentration of an osmo-regulating agent selected from the group comprising polyethylene glycol 8000 (PEG8000), mannitol, sorbitol, NaCI or mixtures thereof, preferably at a concentration of 4%; and
c) Selecting the transformed cells from the population based on the ability of transformed cells to survive and grow in the presence of the osmo-regulating agent.
The cells that have this metabolic advantage are able to grow in the presence of the osmo- regulating agent simulating water stress (drought), while the growth of untransformed cells is inhibited.
Polyethylene glycol (PEG) is a polymer produced in a range of molecular weights. In 1961 , Lagerwerff reported that PEG can be used to modify the osmotic potential of nutrient solution in culture and thus induce a water deficit in plants in a relatively controlled and proper manner for experimental drought protocols.
Cells, including plant cells, cannot grow normally in a culture medium in which a relatively high concentration of PEG 8000 (4% or higher) is present, because they are prevented from capturing water and nutrients causing cell death. The method according to the invention is used to select transformed cells. For this purpose, cells are transformed with the nucleotide sequence comprising a sequence encoding a product that promotes the inhibition of endogenous trehalase enzyme. The population of transformed and untransformed cells is then placed in contact with a culture medium containing an osmo-regulating agent, for example, PEG 8000. The transformed cells can be distinguished from the untransformed cells by the presence in its genome of a selection nucleotide sequence encoding a product that promotes the inhibition of endogenous trehalase enzyme, allowing it to survive and grow in a medium with an osmo- regulating agent such as PEG 8000.
A nucleotide sequence that promotes the inhibition of enzyme function may be an endogenous trehalase sequence encoding a protein having an inhibitory effect on the enzymatic activity of trehalase, for example, sequences encoding the roach protein 85kD (Periplaneta americana), or proteins involved in the synthesis of validamycin, trehazolin, trehalostatin, casteligine, suidastestine, etc. and their active modified forms.
Another alternative to alter endogenous trehalase enzyme in the plant is the use of antisense RNA. This strategy consists in introducing into plant cells a genetic construct that produces a sufficient complementary RNA to the endogenous RNA encoding the trehalase enzyme, so to interact with the endogenous transcript of the plant itself, preventing the mRNA translation at ribosomes and degrading the RNA by post-transcriptional gene silencing, by that inhibiting the
translation of the transcript. This is known as gene silencing, either by antisense technology or RNA interference (RNAi), which to date is widely known.
Thus, the nucleotide sequence that promotes the inhibition of endogenous trehalase enzyme can be preferably a nucleotide sequence capable of transcribed that produce a RNA whose sequence is identical or at least partially complementary to the RNA produced by a DNA sequence encoding the endogenous trehalase. This nucleotide sequence able to transcribe confers to the cell with a competitive advantage in the culture medium with an osmo-regulating agent such as PEG 8000. The nucleotide sequence able to transcribe may also be a nucleotide sequence that produces an RNA that is identical to the RNA produced by a sequence encoding the endogenous trehalase.
According to the present invention, the inhibition of the trehalase enzyme is obtained by transforming a plant with the antisense gene of trehalase from a sequence that is partially or completely homologous to the trehalase endogenous gene to be silenced. Thus, the antisense or RNA trehalase interference gene sequence is used for the transformation of the cell, cell population, tissue, or plant will be partially or completely homologous to the gene or its RNA messenger or trehalase endogenous sequence described in the trehalase sequence from alfalfa, identified as MSTRE gene sequence and the maize trehalase sequence identified as ZmTRE.
The present invention describes therefore that the sequence of MSTRE gene of alfalfa trehalase (Medicago sativa), more specifically of gene MSTRE 1 of alfalfa trehalase, can be used to inhibit the expression of trehalase in maize, because the sequence of alfalfa is homologous to other sequences of trehalase of vegetal origin. Similarly, the maize sequence is homologous to trehalase from other plants to be used in silencing events of the gene encoding trehalase. It is looked at that the sequence of alfalfa or maize can be used in other species, considering the fact that a 100% homology is not required to silence the gene. Furthermore, it is sufficient to express only part of the homologous gene in antisense orientation or an RNA interference to achieve effective inhibition of the expression of endogenous trehalase.
The isolated cDNA encoding the enzyme that degrades trehalose or part thereof, and nucleotide sequences that transcribe antisense RNA or interference RNA subsequently fuse to a promoter sequence so that transcription results in the synthesis of an antisense messenger RNA (mRNA) or an interference RNA (RNAi).
It is understood as interference RNA (RNAi) the RNA molecules involved in post-transcriptional silencing of a gene by this mechanism, namely, hairpin RNA (hpRNA), small RNA (sRNA), and micro RNA (miRNA).
The techniques used for the transformation of plant cells to carry out the present invention are known to one skilled in the art and may be consulted in any literature pertaining to the subject.
The method of introducing to the plant the expressible sequence encoding a trehalase enzyme inhibitor is not crucial to the present invention, provided that the gene is expressed in plant cells, either in a mono or dicotyledon plant cell.
Processes to enter two or more genes in the same plant can be achieved with any of the following methods known in the prior art, which include, for example:
(a) Transforming the plant with a multi copy construct containing more than one gene to be introduced;
(b) Co-transforming the plant with different constructs simultaneously;
(c) Through subsequent rounds of transformation of the same plant with the genes to be introduced;
(d) Crossing two plants, where each of which carries a different gene to be introduced in the same plant; or
(e) Combinations of the above methods.
The term "cell" within the context of the invention also includes protoplasts, and the term "cell population" also includes tissues, organs or portions of them, a population of individual cells within a substrate, or a whole organism, for example, a plant.
The term "cell population" means according to the invention a single cell population also cells in tissues, organs or parts thereof, or cells in whole organisms such as plants, where the whole plant or parts of it may consist of genetically transformed cells.
The term "plant" refers to a differentiated multicellular organism capable of carrying out photosynthesis, such as angiosperms and algae.
The term "plant cell" includes any cell derived from a plant and includes undifferentiated tissue such as callus or corolla tumor and plant seeds, propagules, pollen and plant embryos.
The present invention also includes transformed cells that were selected using the method according to this invention, in particular plant cells and plants regenerated from them, and their seed and their progeny.
The method according to the invention is preferably used to select genetically modified plant cells. Examples of plants for which the method can be used according to the invention include fruit crops such as tomato {Lycopersicum esculentum L), mango, peach, apple, pear, banana, melon etc.; field crops such as sunflower, tobacco, sugar cane (Saccharum officinarum L), etc.; small grains such as wheat (Tritricum aestivum L), soybean (Glycine max L), barley, maize (Zea mays L), cotton, etc.; vegetables such as potato (Solarium tubercosum L), carrots, lettuce, pumpkin, onion, etc.; legumes such as beans, lentils, faba beans, and those plants in need to improve their tolerance to drought and/or cold.
Through the method of the present invention, the transformed cells may be able to survive and grow in drought and/or cold conditions, whereto a plant cell cannot survive and grow. Thus, transformed cells according to the present invention can be selected from the total population based on their ability to survive and develop.
Since genetically modified plants are capable of inhibiting at some level trehalase enzyme because of the introduction of the nucleotide sequence of selection, the cell obtained can modify the flow of glucose and survive in a medium that lacks an optimal concentration available for the cell. Genetically modified plants can therefore develop while the development of untransformed plants is inhibited. When the method according to the invention is used for genetic selection of cells in plants, the transformed plants can be identified visually.
Therefore, the method according to the invention provides a simple and environmentally friendly selection system of transformed cells, particularly for genetically transformed cells. The ability of transformed cells to survive and grow in an environment of low water availability annuls the need to add the medium with a selection marker compound such as antibiotics and herbicides. According to the invention, it can be used as a selection nucleotide sequence in genetically transformed cells, any nucleotide sequence encoding a product that promotes the inhibition of trehalase enzyme. Although exogenous nucleotide sequences can be used, such as those belonging to bacteria, yeasts, or insects, it is also possible to use endogenous nucleotide sequences with the advantage of avoiding the introduction of material from another species. It presents a major advantage in that the nucleotide sequence encoding a homologous or complementary RNA to the trehalase sequence, because its expression would cause a post- transcriptional mechanism of gene silencing either by antisense RNA, by RNA interference or by co-suppression, which by preventing the translation of the RNA messenger, either the endogenous or from the transgene, would prevent the production of an additional protein in the plant. Aside from these advantages is the convenient use of inducible or regulated promoters to limit the process of transcription of the selection sequence at the time required to select the cells when these are cultured after being introduced the nucleotide sequence or sequences of interest along the selection sequence.
With this it is possible to create genetically modified plants whereto, for the purposes of the present invention, we will call "cisgenic" when the nucleotide sequences originate from the same species. For this invention the antisense sequence for the maize trehalase enzyme will come from the same species.
The desired cisgen or transgene and the selection nucleotide sequence can be introduced into the cell by transformation using standard techniques known in molecular biology. Although not essential, the transgene and the selection gene may be linked to each other, so that the presence of the selection gene means that the transgene is also present. Optionally, the transgene and the selection gene may be part of the same construct and be introduced into the cell using the same vector.
Since it is necessary that the introduced nucleotide sequences are expressed in the transformed cell, a genetic construct containing the two nucleotide sequences typically contain regulatory sequences that allow the expression of the same, for example, known promoters and
terminators of transcription. Thus, the co-introduced nucleotide sequence will typically be associated with a promoter, which could be constitutive or regulated.
Many cells, particularly cells of higher plants such as Glycine max and Arabidopsis thaliana, have in their genome the encoding genes for endogenous trehalase.
In a desirable embodiment of the method according to the invention, the selection nucleotide sequence comprises the complementary DNA (cDNA) of the MSTRE enzyme of alfalfa (Medicago sativa) or a potion thereof, and ZmTRE maize gen or a potion thereof.
In another suitable embodiment of the invention the nucleotide sequence comprising a sequence complementary to cDNA of MSTRE trehalase enzyme of alfalfa (Medicago sativa) or a portion thereof, and ZmTRE maize gen or a portion thereof.
In still a further embodiment of the method of the invention the transformed cells can be selected using a combination of positive selection and negative selection. Therefore, the nucleotide selection of interest is co-introduced in the transformed cells with a nucleotide sequence aside from the selection sequence, which encodes resistance to at least one member selected from a group comprising toxins, antibiotics and herbicides, and the medium in or on which the transformed cells have existence.
Although for technical reasons the expression units, that is, the promoter and MSTRE gene or the promoter and ZmTRE gene, must be propagated in a bacterial vector, for example in Escherichia coli, the expression unit is isolated from the vector by obtaining it from PCR or digestion with restriction enzymes at the ends. Once the expression unit or DNA containing the promoter and antisense trehalase gene is obtained, it is used in genetic transformation. Thus, the plants generated lack undesirable sequences present in the bacterial vector, such as antibiotic resistance, replication origin, etc.
The present invention is illustrated in the following examples, which are not intended to limit the scope of the invention, as technical specialists in the field will appreciate that certain modifications to the invention can be done without changing the original scope and spirit.
Example 1. Materials and methods.
a) Nucleic acid manipulation.
Plasmid DNA. Isolation procedures, restriction, ligation and transformation of bacterial plasmid DNA were performed according to standard protocols (Sambrook et al., 1989). Regarding the ligation of PCR products in the vector TOPO pCR2.1® (Invitrogen, Inc.), they were performed according to the manufacturer's recommendations. The sequencing of the fragments obtained by RT-PCR, 3'RACE and GeneRacer® was performed from a small-scale preparation.
Plant DNA. For cloning the promoting sequence rd29A of Arabidopsis thaliana a phenokchloroform extraction of genomic DNA was performed from Arabidopsis plants of 10 days of germination in MS medium with 0.7% agar.
Plant RNA. For cDNA cloning of trehalase gene from alfalfa, we proceeded to a total RNA extraction of tissue from seedlings of alfalfa (M. sativa cv. CUF-101 ) of 7 days of germination in vitro on MS medium with 0.8% agar using TRI Reagent® (Molecular Research Center, Inc.) according to manufacturer's instructions. cDNA synthesis of maize was performed using PCR assembled with large complementary oligonucleotides.
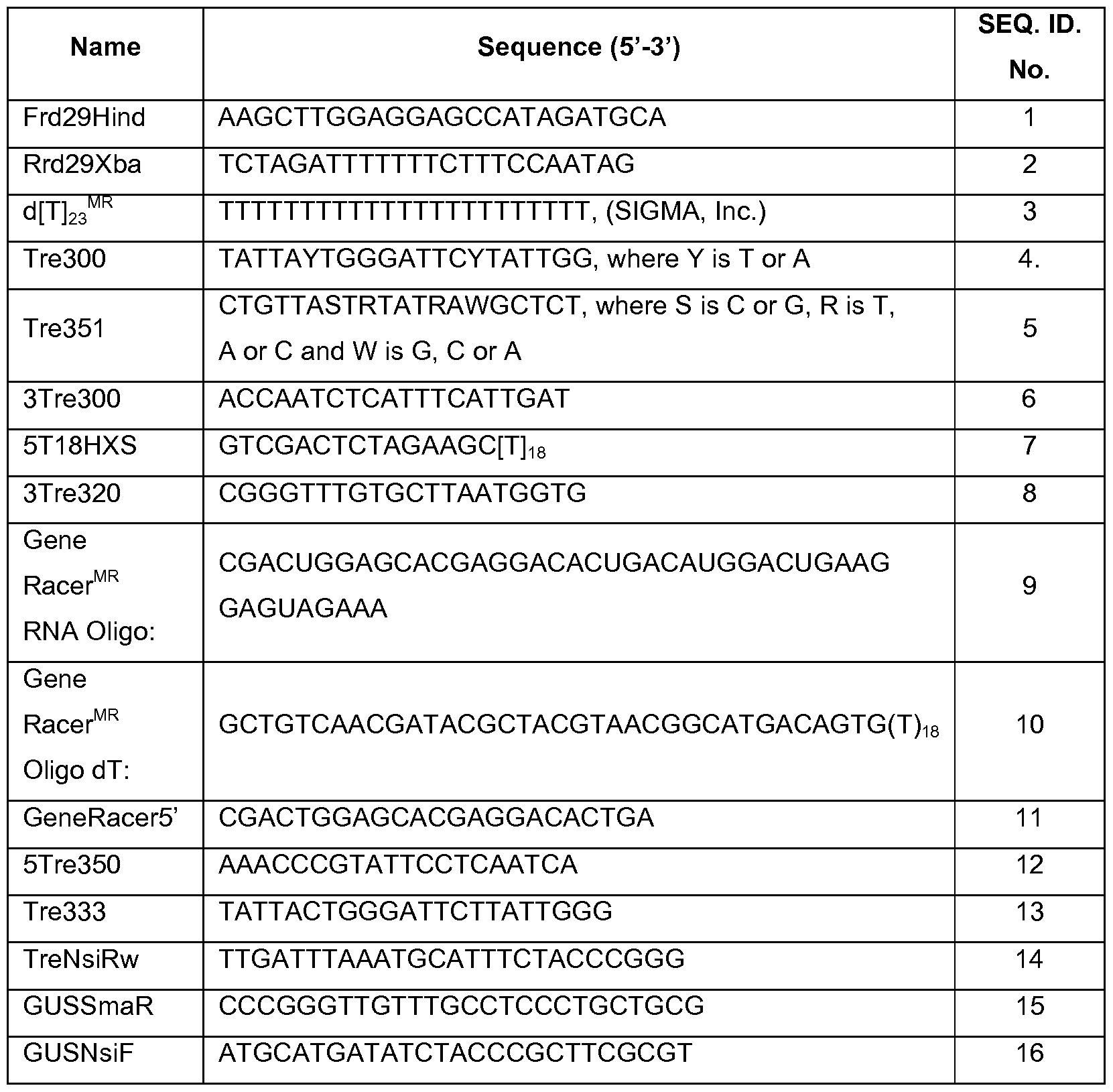
Primers. Following primers were used:
b) Biological material.
Plasmids. For the ligation of PCR products we used vectors pGEM-T Easy Vector® (Promega, Inc.) and TOPO TA pCR2.1® (Invitrogen, Inc.). For constructing the expression vectors for plants we used the vector pBI-121® (Clontech, Inc.). Bacterial strains. The plasmids obtained from the fragment ligation, were introduced by heat shock into calcium competent cells of E. coli strains DH5a, JM109, or from One Shoot ® TOP 10F' (Invitrogen, Inc.) commercial strain.
Plant material. For the extraction of alfalfa RNA, alfalfa seeds from CUF-101 variety were germinated in MS medium with 0.7% agar. For the extraction of Arabidopsis DNA, Arabidopsis seeds were germinated in MS medium with 0.7% agar. For the bombardment of tobacco we used tobacco leaf explants Nicotiana tabacum L. cv Xhanti growing axenically in MS medium with 0.7% agar supplemented with sucrose at 2%. The cultures were maintained under cycles of 16 h light / 8 h darkness at 26°C, and subcultured after 30-40 days in fresh medium.
Example 2. cDNA cloning of the MsTRE alfalfa gene.
As there is no report of the sequence encoding trehalase from alfalfa, which we call MsTRE (Ms: Medicago sativa; TRE: trehalase), and to obtain the fragment used in the constructs to inhibit this enzyme, we proceeded to obtain the complete sequence of its DNA. We used therefore; protocols based on the amplification of this gene transcript (RT) and its subsequent PCR amplification (RT-PCR). a) Amplification of an internal fragment by RT reverse transcription and PCR (RT-PCR)
For the amplification of cDNA internal fragments, simple tests of RT-PCR were performed using the degenerate oligonucleotides Tre300 and Tre351 , which were designed based on a conserved region among the reported encoding sequences in the database (Entrez, NCBI) for Arabidopsis trehalase enzyme (Arabidopsis thaliana; access AAF22127), potato (Solanum tuberosum; access A67882) and soybean (Glycine max; access AAD22970). The alignment of the sequences was done using Clustal method (Software DNA Star).
For the reverse transcription reaction (RT) we used the Enhanced Avian HS RT-PCR system (SIGMA, Inc.), and we required ^g of total RNA of good quality alfalfa, which was incubated at 70°C for 10 min with 1 μΙ_ of Anchored Oligo (dT)23 ® (Ο.δμς/μΙ.) and 1 μΙ_ of a mixture of dNTPs (dideoxynucleotides) (10 mM each) in a final volume of 10 μΙ_ with sterile bi distilled water, according to manufacturer's instructions. The mixture was placed on ice immediately after incubation in heat, and 6 μΙ_ of PCR reagent grade water, 2 μΙ_ of AMV-RT 10X Buffer, 1 μΙ_ of RNAse inhibitor (201Ι/μΙ_) and 1 μΙ_ of RT Enhanced AMV enzyme (201Ι/μΙ_) were added, in a final volume of 20 μΙ_. This mixture was incubated at 42°C for 60 min to allow the synthesis of the first strand of DNA.
For the PCR amplification we used 3 μΙ_ of the first strand synthesized by RT, 1 μΙ_ of DNA Polymerase JumpStart AccuTaq® (2.51Ι/μΙ_), 5 μΙ_ of AccuTaq® 10X Buffer, 1 μΙ_ of dideoxynucleotides mixture (10 mM each), 2 μΙ_ of degenerate sense oligonucleotide Tre300 (100 ng/μΙ.) and 2 μΙ_ of reverse degenerate oligonucleotide Tre351 (100 ng/μΙ.) in a final volume of 50 μΙ_ PCR reagent grade water. With this reaction mixture, we performed PCR to amplify an internal fragment of trehalase cDNA.
The fragment obtained by PCR was cloned into pGEM-T Easy Vector®, which was subsequently introduced by heat shock of 45 sec at 42°C in calcium-competent DH5a bacteria.
The transformed bacteria were characterized by EcoR\ enzyme digestion of plasmid DNA, and we selected those that released the amplified fragment. Sequencing of this fragment gave us the nucleotide sequence which we designed the oligonucleotides used in subsequent protocols for cloning 5' and 3' cDNA ends of the alfalfa trehalase. b) Amplification of the 3' end by 3'RACE protocol.
For the 3'RACE protocol, we used the first DNA strand initially synthesized by reverse transcription. With this cDNA we performed an initial PCR amplification using the specific oligonucleotides 3Tre300 and 5T18HXS which were designed from the sequence obtained for the internal fragment. This was followed by a semi nested PCR using the oligonucleotides 5T18HXS and 3Tre300, being this last designed from an internal position of the sequence obtained for the internal fragment. The amplified sequence was cloned into a TOPO TA pCR2.1® vector (Invitrogen, Inc.), resulting plasmid TOPO-3Tre, which was purified for Eco Rl enzymatic characterization and subsequent sequencing and manipulation. Because most of the conserved regions between the sequences of plant trehalase are found in this region, this fragment is sufficient to design the vectors to inhibit endogenous trehalase enzyme. c) Amplification of the 5' end by GeneRacer.
For obtaining the 5' end of the alfalfa trehalase cDNA we used the GeneRacer® system. In this protocol we started from 3 μg of total RNA earlier handled, following the manufacturer's instructions for RNA dephosphorylation, CAP removal structure and its subsequent binding to the provided RNA oligo. From this modified RNA messenger we proceeded to the synthesis of the complementary strand (cDNA) using the AMV-RT® system (SIGMA, Inc.). The obtained complementary strand was subsequently used as template in PCR amplification using oligonucleotides GeneRacer5'® and the reverse oligonucleotide 5Tre350 designed from the sequence of the internal fragment obtained by RT-PCR. The amplified sequence was cloned into vector TOPO TA pCR2.1®, resulting in plasmid TOPO-5Tre, which was purified for enzymatic characterization with Eco Rl endonuclease and for subsequent sequencing.
From the alignment of the three fragments obtained by this strategy, we obtained the complete sequence of the cDNA of the alfalfa trehalase (MsTREI ).
Example 3. Design of antisense TRE sequences (TREas) and interference RNA (Tre-RNAi) a) Amplification and cloning of TRE internal fragment with greater homology between species. For expressing in the plants a trehalase antisense sequence under promoters 35S and rd29A, we first selected a fragment (SEQ. ID. No. 18) comprises a 560 bp internal fragment of the sequence, which includes small regions conserved between the different plant trehalases. To do this, we designed a pair of specific oligonucleotides Tre333 and TreNsiRw that amplify the internal fragment (TRE) selected from DNA plasmid of vector TOPO-3Tre. The TreNsiRw oligo
introduces an Xma I site followed by an Nsi I site into the 3' end of the sequence. The amplified product was cloned into vector TOPO TA pCR2.1®, resulting in plasmid TOPO-tre560s and TOPO-tre560as due to the fragment bi-directional insertion into the vector. In these two constructs the insert is flanked at one end by Sad site and on the other side by the Xba I and Nsi I sites of the vector, which allowed us to determine the direction of the fragment by digestion in site Nsi I of TRE sequence with respect to these sites (sense direction: Sacl-Xbal; antisense direction: Xbal-Sacl). b) Construction of the intermediate vector TOPO-GUS.
For constructing RNA interference for alfalfa trehalase it was necessary to have a "loop" sequence to join both copies of TRE sequence. We used an internal fragment of 1 ,020 bp of the gene sequence of b-glucoronidase (GUS; gene uidA) of E. coli. This sequence was amplified by PCR using the GUSSmaR and GUSNsiF primers from plasmidic DNA of pBI-121 plasmid. The primers used were designed based on GUS sequence included in the sequence of vector pBI- 121 (AFA85783 access) and inserting into the ends of the fragment an Xma I site and an Nsi I site. The PCR product was cloned into vector TOPO TA pCR2.1®, resulting in plasmid TOPO- GUSs and TOPO-GUSas due to the fragment bi-directional insertion into the vector, which was determined by Nsi I digestion. c) Construction of RNA interference with TRE fragment.
For constructing the RNA interference for trehalase, we placed two copies of fragment TRE in opposite direction to each other (head to head) and linked them to the amplified fragment of 1 ,020 bp of GUS sequence. For this, fragment TRE of plasmid pTOPO-tre560as was initially released as fragment Xba \-Nsi I and inserted into the same sites of plasmid pTOPO-GUSs, resulting in plasmid pTOPO-GT (GUS:TRE). Subsequently, GT fragment (GUS:TRE) was released from this plasmid by Sac\ -Xma\ digestion and cloned into the same sites of plasmid pTOPO-GUS AS, resulting in plasmid pTOPO-TGT (TRE:GUS:TRE). The positive clones for this construct were characterized by Sac\-Xma\ double digestion and by Nsi\ simple digestion. Example 4. Construction of control expression vectors.
a) Amplification of rd29A promoter sequence.
Promoter sequence rd29A was amplified by PCR from the genomic DNA extracted from leaf tissue of Arabidopsis seedlings. The seeds were provided by the Institute of Biotechnology, UNAM-Cuernavaca. For the amplification, we designed the oligo primers Frd29Hind and Rrd29Xba based on the reported promoter sequence (D13044 access). This pair of oligos amplifies the fragment comprised from nucleotide 4563 to 5504 and is flanked by sites Hind III and Xba I. The PCR product was cloned into vector TOPO TA pCR2.1® to produce plasmid TOPO-rd29.
b) Construction of inducible expression control vector in plants: prd29A:GUS.
Promoter sequence rd29A was released from vector TOPO-rd29 by double enzymatic digestion with Hind \W-Xba I and subcloned into the same sites of vector pBI-121® (Clontech, Inc.)- In this construct, sequence rd29A replaces promoter 35SCaMV, resulting in plasmid prd29A:GUS. This vector is the control plasmid of inducible expression vectors for antisense and RNA interference of trehalase. c) Constitutive expression control vector in plants: pBI-121 (p35S:GUS).
Promoter sequence rd29A was released from vector TOPO-rd29 by double enzymatic digestion with Hind III and Xba I and subcloned into the same sites of vector pBI-121® (Clontech, Inc.). In this construct, sequence rd29A replaces promoter 35S CaMV, resulting in plasmid prd29A:GUS. This vector is the control plasmid of inducible expression vectors for antisense and RNA interference of trehalase.
Example 5. Construction of expression vectors for TRE antisense fragment (TREas) and trehalase RNA interference (TRE-RNAi).
a) Construction of the expression vector of TRE antisense fragment (TREas) under promoters 35S and rd29A.
For directing the trehalase sequence in antisense direction in plasmids with promoters 35S and rd29A, we released the trehalase fragment of vector TOPO-tre560s by Sac \-Xba I digestion and subcloned it into the same sites of plasmids pBI-121 and prd29A:GUS, resulting in plasmids p35S:TREas and prd29:TREas. In both vectors, the antisense fragment replaces GUS gene and precedes the NOSter region (termination and polyadenylation region of the gene encoding nopaline synthase). b) Construction of the expression vector of the RNAi-TRE cassette under promoters 35S and rd29A.
For the expression in plants of trehalase RNA interference (TRE-RNAi), this cassette was released from vector TOPO-TGT by Sac \-Xba I double digestion and subcloned into the same sites of plasmids pBI-121 and prd29A:GUS resulting in plasmids p35S:TRE-RNAi and prd29:TRE-RNAi. In both vectors, RNAi cassette replaces GUS gene and precedes the NOSter region (termination region of nopaline synthase gene).
Example 6. Obtaining GMs maize plants according to the invention.
a) Integrating into maize the constructs for the expression of fragment TRE antisense (TRE AS) under 35S and rd29A promoters.
For obtaining GMs maize plants of B73 and H99 inbred lines that express the antisense of trehalase enzyme controlled under 35S or rd29A promoters, plasmids p35S-TREas and
prd29A:TREas were integrated by biolistic and with the use of embryogenic callus growing in axenic culture. b) Preparation and micro-particle bombardment of DNA.
For the bombardment of DNA/particles, we used the high-pressure Helio PDS 1000-He® system. The preparation of tungsten particles, DNA coating and bombardment parameters were performed according to known protocols using 6 boxes for each DNA construct bombarded.
Example 7. Evaluation of the system as a selection marker.
A selection was made in culture medium with growth regulators to induce the formation of somatic embryos (embryogenic callus) supplemented with PEG-8000 at 4% with embryogenic callus bombarded with trehalase antisense constructs and its controls, maintained in MS culture medium supplemented with 2 mg/L Dicamba, 40 mg/L adenine, 3% sucrose and 4% PEG 8000, and incubated at 26°C and a photoperiod of 16 h light / 8 h darkness.
Example 8. Construction of a maize trehalase expression unit.
We selected the gene encoding maize trehalase enzyme to generate a cisgenic plant. We reversed this open reading frame (ORF) and obtained the supplementary to express it under simple promoter 35S regulation with the polyadenylation sequence of the gene encoding the nopaline synthase enzyme from Agrobacterium tumefaciens. The obtained sequence was modified in two bases to eliminate two internal EcoRI restriction sites; we also inserted a non- encoding sequence that would serve as Tag to identify this transformation event. It must be noted that the antisense gene does not contain any open reading frame, so there is no protein synthesis to reverse this ORF. There is a natural asymmetric restriction site for EcoRV, which serves as reference in detection assays through other methods (figure 1 ).
This expression unit was obtained by mating long complementary oligonucleotides in vitro assisted by PCR. The produced fragment of 1802 bp was cloned into a bacterial vector pCR8 Topo (Quiagen). Digestion with EcoRI produced a fragment of 1.8 Kb, which was purified and used in biolistic experiments on embryogenic maize callus.
Example 9. Genetic transformation of maize inbred line B73 with the use of embryogenic callus and biolistic.
The embryogenic callus of maize inbred line B73 (see figure 2) was induced from mature seeds in MS culture medium (Murashige and Skoog 1961 ) supplemented with 2 mg/L Dicamba, 40 mg/L adenine, 3% sucrose, pH 5.8 and 2.5 g/L gelrite. We used an embryogenic line with high cell division capacity compared with the rest of the lines.
The maize embryogenic callus were bombarded with gold microparticles containing the plasmid with the antisense expression unit of trehalase (for example trehalase of alfalfa according to the
example 3 or B73 maize trehalase according to example 8), and later were selected in the presence of 4% PEG 8000 for two months (three subcultures were performed) to generate PEG-tolerant clones whereas not bombarded callus (referred to as negative controls) showed cell death.
The obtained transgenic clones were propagated during one month in a medium with PEG until the quantity was enough to perform the regeneration of plants in MS culture medium (Murashige and Skoog 1962) supplemented with 0.2 mg/L BAP, 0.1 mg/L kinetin, 1 % sucrose, 2.5 g/l gelrite. The regenerated plantlets were transferred to soil in greenhouse conditions for promoting their life cycle to obtain seeds. The generated seeds were sterilized with chlorinated gas to avoid future contamination with fungi. To monitor the tolerance ability to PEG 8000, the transgenic seeds, T1 generation, were grown in flasks containing 7% PEG 8000, comparing their ability to survive with untransformed (wild) seeds of inbred line B73. Transgenic seeds in T1 generation showed a segregation for their ability to grow at 4% PEG 8000 (25% tolerant), while negative controls could not germinate under these conditions. At this stage, the transformed plants showed their ability to survive in an environment with water deficit. Tolerant plants were transferred to the greenhouse for subsequent generations T2, T3, following the selection procedures in the seed stage with PEG 8000 until generate a population that would produce ears with 100% of PEG 8000 tolerant seeds. Two transgenic lines were selected, which we called T1 and T4 for submission to drought tolerance assessments. These lines showed the best agronomic performance in their life cycle (figure 8).
The plants resistant to 7% PEG 8000 were transferred to greenhouse conditions to enable them to complete their life cycle. DNA was isolated from 5 independent genetic transformation events to verify the insertion of 35S functional unit, neomycin phosphotransferase type II NPTII gene present in the bombarded plasmid; as it is observed in figure 3, T1 and T4 contained the expected fragment. For their growth and vigor capacity, we selected lines T1 and T4 for future drought tolerance experiments.
Example 10. Molecular characterization of drought-tolerant maize plants.
For this phase we used seeds derivate from the third inbred generation of transgenic lines T1 and T4. At this phase, the selection of plants to perform the experiment consisted of germinating the seeds in a solution of PEG 8000 at 7%, which represents a water-stress medium by not allowing PEG available to water for absorption by the seed. The seeds that were able to germinate under this selective agent were subsequently grown in greenhouse conditions and rooms with growth control. We took a segment of 2 cm2 from the plants leaves, from which we extracted the total DNA with a DNeasy kit (Quiagen). This DNA was used as template to amplify the open reading frame of the gene and an internal segment of 35S promoter, using specific oligonucleotides. The products were resolved in 1 % agarose gel, which shows a band of approximately 950 bp on GM plants that corresponds to the expected size. As a control we
used DNA from control plants, while as negative control we performed a reaction without the addition of DNA template (figure 4).
Example 11. Trehalase transcript detection by quantitative RT-PCR (RT-PCR in real time). The molecular strategy of gene attenuation was performed using the expression of antisense open reading frame of the gene encoding the trehalase enzyme. Because this strategy does not extinguish the transcription of the reference gene, we calculated the silenced proportion by quantifying the accumulation of this gene. Figure 5 shows that in drought conditions the attenuation of the expression is evident through a lower accumulation of the reference transcript in 86% and 76% of the higher value presented by the plants when grown under normal irrigation. This attenuation consisting of a decrease in mRNA accumulation between 14% and 24% is sufficient to provide a phenotype of tolerance to drought stress in GMs plants.
Example 12. Quantification of total glucose as a measure of trehalase enzyme activity. Trehalase is an enzyme that uses trehalose as substrate, and in the presence of water it produces two glucose molecules. We used the method described by Freydiere et al. (2002) adapted to maize. 100 mg of fresh tissue was macerated in a micro tube using a pestle. The homogenate was centrifuged, and the supernatant was transferred to a clean tube. We used Roche's electronic micro cell device of Accu-chek, pre calibrated for glucose. The glucose values obtained from wild plants were 64 mg/dL, whereas GM plants expressing antisense trehalase showed values of 51 mg/dL. The attenuated trehalase by molecular strategy prevents the hydrolysis of glucose from trehalose, being consistently quantified in 79% of the concentration determined in wild plants. These results are consistent with the accumulation of mRNA detected by quantitative RT-PCR at real-time.
Example 13. Physiological characterization of drought-tolerant maize plants. a) Growth of B73 lines GMs. Evaluation and selection of seeds.
Maize seeds from cisgenic lines B73-T1 and B73-T4 from the invention and B73 wild maize as control, Creole black and white, and white and cacahuazintle maize were put to germinate in petri dishes with water in a grow chamber at 26°C for 5 days. After germination, seedlings were transferred to a substrate containing peat agrolite sandy soil in 60cm deep and 25 cm diameter PVC tubes. The bottom cover contained many holes to allow water draining; this cover can be removed to observe the root portion. Finally, we performed the tracking of vegetative growth, apparition of anthers and female flowers, seed production and senescence.
b) Measurement of agronomic parameters in vegetative phase (figures 6 and 7).
As internal comparison-control we used two varieties of creole maize known for their drought- tolerance capacity. Two months after sowing the maize, the Creoles showed a greater height for the transformed lines, the smallest being the B73 control. Significantly, the size of creole maize is a genetic characteristic of the Creoles of reference, which is evident in any habitat. Figure 8 shows the phenotypes obtained from vegetative growth under irrigated conditions.
In the images of figure 8 we see clear growth differences among the creole varieties, which are larger; the next in size are the transformed B73 T1 and T4, and the smaller ones are the B73 control.
The drought was applied simply by stopping the supply of water and mineral solution. As indicated, the substrate is composed of peat and agrolite, which have low water retention, showing the effects of drought on the phase of increased susceptibility of the plant, which is the production stage of male and female flowers, and number of ears and grain filling. The drought effect is observed in figures 8 to 10 on this phenologic stage. c) Flowering: male flower (anthers and pollen).
The first plants to produce spices, which are components of the male flower were B73 T1 and T4 compared to control plants B73, which produced them 25 days later. The appearance of spikes in creole varieties occurred 30 days later. This delay in flowering is characteristic of plants with water stress and an increased range of occurrence of spikes and cobs (figure 9). d) Flowering: female flower (ears).
The first plants to induce the formation of female flowers were lines B73 T1 and T4, compared to the control 17 days later. Very noticeably, the wild plants creole white and black though producing spikes, they did not show the formation of female flowers or ears, presumably because of the stress they were subjected to. After grain filling the plants entered the stage of senescence, which is characteristic of a decrease in photosynthesis and turgor in the plant (figure 10). e) Seed production.
Seed production was evaluated by collecting the ears of each plant analyzed and those that developed them. Previously to the evaluation, they were properly dried at 37°C for 8 days. As seen in table 1 , there is a marked increase in the seeds of transformed plants compared with the negative control. The number of seeds per row and the number of rows and average weight indicate a marked superiority of the plants of the invention to produce seeds. The results are the average of 15 ears per genotype.
Table 1
The state of senescence was comparable in all treatments, regardless of the formation of female flowers.
Example 14. Agronomic patterns measuring. a) Growth measuring.
Figure 1 1 shows the plant height, measured from its base to the apical growth zone; in kinetics from seed germination to senescence. The plotted points show the averages obtained from plants of each treatment. As we can see, the BT73 line plants showed a lower growth rate than the Creoles. However, the comparison of BT73 GM and control showed a higher rate in the GM. Creole varieties showed a greater growth, and it was very similar among them; they surpassed 3.5 meters height. It should be emphasized that the line B73 control increased its growth before it started blooming, since it had a greater expenditure of nutrients thereat and use them to grow and flourish simultaneously, a situation contrary to transformed plants, which were synchronized, growing first and after a while flourished. As indicated, Creole plants stressed by drought failed to produce ears, despite having reached great hights. b) Physiological comparisons.
Regarding the physiological comparison between varieties, in figure 12 we can observe the differences in size, color of the leaves and stem, where Creole varieties show an increased size compared to B73, and its color is pale green and less bright than those of B73 that have a strong and lustrous green, and its leaves are fleshier. Concerning the shape and thickness of the stem the Creole have a thinner and round stem, and the stem of B73 tends to be oval and thick, offering more resistance and the possibility of having a fully erect growth unlike the Creoles, where the stem begins to bow after reaching a height of two meters. It is necessary to perform a field test, as the secondary root of corn is limited by controlled greenhouse tests.
Example 15. Measurement of photosynthetic parameters under conditions of humidity and drought-stress.
a) Drought induction.
The plants were divided into two groups; the irrigation in one group was stopped, while in another it was maintained as control. Figure 13 shows the stress phenotype displayed by the plants after stress, and we can observe the characteristics after 13 days of stopping irrigation.
In figure 13, we can observe the response of plants to drought stress compared to plants that were not subjected to this treatment. The black and white Creole varieties showed a greater drought stress and presented the loss of turgor on their leaves, wilting and chlorosis; these effects were stronger in black Creoles. The drought had to be suspended on creole plants 6 days after starting its induction, because they already showed severe effects of stress damage.
The plants that remained without water after this time reached a permanent wilting point.
Effects were observed only 1 1 and 13 days after inducing drought for cisgenic plants; their leaves presented a curl and mild chlorosis. The plants showing a greater effect were T4, and they also slowed their growth during drought action. About the B73 plants, they did not show noticeable effects, because thereat their longitudinal size was significantly lower to the transformed plants, which conferred them a lower water requirement. b) Photosynthetic parameters.
B73-T1 line had a higher photosynthetic rate in wet conditions compared to control varieties and T4; in contrast, during drought all varieties decreased their photosynthesis at 6 days, but only T1 showed a slight increase at 13 days where plants were irrigated (figure 14). The last bar of each series showed in figure 14 indicates the photosynthetic rate at which the varieties return after starting the irrigation.
Conductance is a measure of the concentration of ions in plants, whether intra- or intercellular. While measuring the conductance, GM T1 plant showed a response of decreased conductance greater than 50% at 6 days and more than 100% at 13 days of drought, compared to T4 plant, which decreased this conductance nearly 50% at 6 days and the control only at 13 days, where it decreased approximately 40% (figure 15). This leads us to conclude that T1 shows a higher survival response and water consumption than T4 and Creoles.
Figure 16 shows the internal water content of the plants under test, where we can observe that T1 has the lowest water content to T4 and drought control. T1 and T4 show a marked adaptive response, because they can store significantly more water than control plants after a drought- stress regime.
An important parameter is the relative humidity of the soil where the plant grows. To corroborate the response to the lack of water, we measured relative humidity in plant and the soil, and we observe that control plants started the drought period even with more water than GM plants. Subsequently, the relative water content was decreased by treatment effect. At 13 days the
roots had absorbed 50% of water in the pot as opposed to the control plants, something which may happen because control plants had a smaller size at the time of comparison and their water requirements are lower (figure 17).
The measurement of intracellular C02 reflects the stomatal opening, which is influenced by drought. The opening of the cells that allow gas exchange is controlled by a mechanism dependent on abscisic acid, which is caused by water stress and other abiotic stress factors. Figure 18 shows that despite applying the stress, the GM plants of the invention continue to exchange gases, in contrast to the control that remains with high internal C02 concentrations. The above idea can be corroborated in figure 19 showing the stomatal opening at the time of measurement, where we observe that it is true that T1 has a lower respiratory rate, since it closes its stomata more than 50%, while the response of T4 at the beginning of drought is delayed; but after 6 days, it decreases its transpiration rate more than 50% compared to control B73, which only decreases its respiratory rate at 6 days to more than 30%, and at 13 days to almost 50%.
Example 16. Cold-stress tolerance in plants with antisense trehalase.
Control maize plants and with the antisense trehalase gen obtained according to the present invention were germinated at 4°C for three days and were subsequently grown at 16°C. Wild plants could not progress in their growth, in contrast to GM of the invention, which were transferred to pots for the progression of vegetative growth. In controlled greenhouse conditions, the temperature fluctuation in winter was allowed up to 2°C as minimum temperature. The plants of the invention with antisense trehalase gene progressed in their vegetative growth, in contrast to wild plants. In vitro and greenhouse data are consistent, in that plants with improved antisense trehalase gene according to the present invention are cold tolerant. Also, the plants of the invention obtained were able under these conditions to develop the first spikes, which are the male organ pollen generators to start their reproductive stage. There are three possible mechanisms that may explain the protective ability of trehalose on biomolecules, such as the availability of water, crystal formation, and chemical stability. These are not exclusive and may have additive effects to produce the observed tolerance to drought and cold generated according to the present invention. For example, the hydration shell formed by water interacting with the molecule via hydrogen bridges can stabilize the molecules and inhibit irreversible denaturation (Roser et al., 1993; Clegg, 1985).
The glycosidic bond between two residues of D-glucose shows chiral flexibility, probably allowing trehalose to interact with other polar groups of different molecules. Trehalose is the only sugar that forms stable amorphous crystals in extreme temperatures. This disaccharide has been proposed as a protector of enzyme activity, stabilization of enzymes, food additives, cryopreserved cells, tissues and organs, and improving the shelf life of flowers (Eroglu et al., 2000; Guo et al., 2000).
This disaccharide has also been described as a signaling molecule. Our evidence allowed us to quantify trehalose in the phloem sap, where the enzymatic reaction for its synthesis is compartmentalized. The producer or photosynthetic tissues contain trehalose phosphate synthase enzyme, while phosphate phosphatase trehalose is found in consumer tissues.
Example 17. Quantification of trehalose by HPLC.
Samples were analyzed using liquid chromatography with a refractive index detector, using three samples per treatment from control B73, B73 T1 and B73 T4; during the separation of the components in the extract injected, the peak corresponding to trehalose is obtained in approximately 17 retention time, this based on what was obtained from standard commercial trehalose, although there resulted other peaks that correspond to the disaccharide of interest, some of which correspond to glucose and sucrose, and others not identified (figure 20).
- Trehalose content in plant samples.
For the quantification of trehalose, from figure 20 we calculated the area under the curve (Δ) and the biomass in mg [□], both from the sample (mtra) and the standard (std), where a biomass of the standard of 0.25 mg was used and a plant sample of 100 mg, using formula (1 ), and obtaining the results in table 2 for samples under test:
%Trehalose = (Amtra/Astd)([n]std/[n]mtra)(100) (1 )
Table 2
Astd = 280950
[□]std = 0.25 mg
[□]Mtra = 100 mg
According to the above, the values of fresh weight trehalose were obtained from the analyzed samples; in table 3 these values are compared with those reported on this disaccharide in wild maize plants.
Table 3. Trehalose concentration (mg/g fresh weight) in samples of B73 control and genetically modified according to the invention, as analyzed by HPLC
As shown, the value obtained in control plants was similar to that reported earlier in maize plants, and it would be equivalent to 100% of the total of that of B73 maize plants, so the values found in T1 and T4 genetically modified plants according to the present invention would amount to an accumulation of at least 68% and 74% higher of trehalose compared to the control; these results extend beyond the ranges reported and indicate that the inhibition of the trehalase enzyme was achieved (figure 21 ).
Example 18. Measurement of the enzymatic activity of trehalase.
The quantification of the enzymatic activity of trehalase was performed by the quantification of glucose hydrolysis of trehalose. This was done to verify the results obtained in example 17 (table 4). An enzyme unit is equal to 1 μηηοΙ/Ι of trehalose.
Table 4. Glucose levels and trehalase activity detected in extracts of control and genetically modified B73 plants (T1 and T4)
* = 1 U trehalase
By this experiment it was observed that the genetically modified plants of the invention did not produce glucose with respect to B73 control plants (figure 22), which indicates that the attenuation of trehalose action in trehalose was achieved, confirming the data obtained from the trehalose levels preset in genetically modified plants (T1 and T4). Example 19. Marking LD-PCR (Long-Distance PCR).
After obtaining the cDNA previously synthesized from total RNA of maize leaves, we proceeded to perform a marking LD-PCR assay using 32P-dCTP, using samples both in drought and wet conditions of control B73, T1 and T4, in a total of 6 samples that would be used in macroarrays;
at the time of testing we also performed a test without markup as a template of the cDNA of T4 (in wet conditions) to verify that the reaction mixture and amplification conditions were correct (figure 23), which was observed by gel electrophoresis at 1 % agarose at 80 volts and visualized in photo-documenter Gel Logic 200 (Imaging System).
Example 20. Quantification of differential expression using a macroarray.
With the cDNA that was used as template for the synthesis of radioactive probes according to example 19, they were hybridized with the plasmids shown in table 5, blotted onto nylon membranes containing the DNA fragments shown in table 5. The mentioned plasmids come from the construction of differential expression answer genes libraries to drought reported by Montalvo-Hernandez (2007) and Barrera-Figueroa (2007), as well as plasmids that contain answer genes to drought in beans not published.
Table 5
No. at gel Key GEN No. at gel Key GEN
Chloroplast precursor
2 PV1 Unknown 19 PV21
small HSP
3 PV2 Unknown 20 PV22 Hypothetic protein
4 PV3 Dehydrine 21 PV23 Phaseolus vulgaris est
Answer to Carboxyl transferase
5 PV4 22 PV25
humidity cDNA a subunit accA
6 PV5 Taumatine protein 23 PV26 Thioredoxine
Intrinsic protein
7 PV6 24 PV27 Unknown
of tonoplast
8 PV8 Cold induced 25 PV28 Unknown
Prophyline
9 PV9 26 PV29 Unknown
(Phaseolus vulgaris)
Protein kinase SPK-3
10 PV10 27 PV30 Unknown
(Phaseolus acutifolius)
1 1 PV1 1 Hypothetic protein 28 L2 Beta galactosidase
Probably senescence
12 PV12 Hypothetic protein 29 L6
protein
Chloroplast Transmembranal amino acid
13 PV13 30 L8
phosphoglucomutase transport protein
Chloroplast hypothetic
14 PV14 Chloroplast protein 31 L13
protein (Zea mays)
Asparagine synthetase
15 PV16 Hypothetic protein 32 L14
(Phaseolus vulgaris)
Mio-inositol-1 -phosphate
16 PV17 Hypothetic protein 33 L15
synthase
LEA group 4
17 PV20 Unknown 34 L17
(Phaseolus vulgaris)
Table 5 (cont.)
The plasmids of table 5 were dripped in a total of 400 ng (2 μΙ: 1 μΙ plasmids + 1 μΙ NaOH) were blotted onto a nylon membrane for the macroarray of DNA; these membranes were then hybridated with radioactive probes obtained from cDNA radiolabelling of RNA synthesized in drought and wet conditions, and were exposed to X rays at -80°C and developed the next day, visualizing different intensities of hybridization signals (figure 24). The obtained signals were analyzed using Array Vision software (GE), subtracting the background around each signal and finally obtaining the intensity associated to each of them.
Example 21. Quantification of gene induction.
Table 6 shows the signal intensity values obtained in control samples B73, T1 and T4 according to example 20, and the subtraction of both values about their background, indicating the induction level of signals; for genes in which the subtraction is zero, this indicates that this gene was not detected since the subtraction was performed to know whether there was an induction or repression of genes in drought conditions, according to the present invention. Zero values indicated that these genes had an expression out of the detectable threshold according to the test.
Table 6. Differential expression values calculated from hybridization assays from over- regulation coefficient [R= Intensity Drought (stressGd) / Intensity Humidity (irrigated) * 100 ] of plant sample from leave of B73 control, T1 and T4 plants under drought conditions
B73 CONTROL B73 T1 B73 T4
KEY
HUM DRO SUBST HUM DRO SUBST HUM DRO SUBST
PV1 Unknown 75 86 12.8 82 61.5 -33.3 69.5 51 -36.3
PV2 Unknown 28.5 28.5 0.0 19 9.5 -100 12.5 55 77.3
PV3 Dehydrine 79 74 -6.8 79.5 85.5 7.0 66 51 -29.4
PV4 Humidity response 77.5 78 0.6 81 90 10 67 51 -31.4
PV5 Taumatine 74.5 81 8.0 78 81.5 4.3 61.5 51.5 -19.4
PV6 Aquaporine 81 78 -3.8 54.5 43 -26.7 22 53.5 58.9
PV8 Cold induced 83.5 84.5 1.2 77.5 38 -103.9 48 45.5 -5.5
PV9 Prophyline 80.5 70 -15.0 81.5 82 0.6 61 47 -29.8
PV10 Kinase SPK3 21.5 10.5 -104 0 0 0 3 23 87.0
PV1 1 Hypothetic protein 15 7.5 -100 0 0 0 1.5 17.5 91.4
PV12 Hypothetic protein 81.5 78.5 -3.8 53.5 28 -91.1 40.5 54 25
PV13 Phosphoglucomutase 77.5 74.5 -4.0 82 77 -6.5 62.5 54.5 -14.7
PV14 Chloroplast protein 33.5 29.5 -13.6 10 3.5 -185.7 9 47.5 81.1
PV16 Hypothetic protein 75.5 72.5 -4.1 74.5 85 12.4 65.5 55.5 -18.0
PV17 Hypothetic protein 2 0 0.0 0 0 0 0 1.5 0
PV20 Hypothetic protein 81 81.5 0.6 84.5 56 -50.9 68.5 59 -16.1
PV21 Hsp precursor -6 0 0.0 0 0 0 0 6.5 0
PV22 Hypothetic protein 4 0 0.0 0 0 0 0 21.5 0
PV23 Hypothetic protein 84 78.5 -7.0 81.5 85.5 4.7 56.5 58 2.6
PV25 Carboxyl
-1 0 0 0 0 0 0 0 0 transferase a
PV26 Tioredoxine 1 0 0 0 0 0 0 0 0
PV27 Hypothetic protein 1 1.5 0 0 0 0 0 0 8.5 0
PV28 Hypothetic protein 7.5 0 0 0 0 0 0 10.5 0
PV29 Hypothetic protein 39.5 27.5 -43.6 9 0 0 5 62 91.9
PV30 Hypothetic protein 83 72 -15.3 20 1 1 -81.8 100 64 72.7
L2 Beta galactosidase 1 0 0 0 0 0 0 3.5 0
L14 Asparagine
2.5 0 0 0 0 0 0 3.5 0 synthetase
L90 Lipid transfer 5.5 4 -37.5 0 0 0 0 14.5 0
L89 BURP dominium
0 0 0 0 0 0 0 14 0 protein
L6 Senescence protein 0 0 0 0 0 0 0 4 0
L13 Hypothetic protein 5 7.5 33.3 0 0 0 0 21.5 0
L19 Hypothetic protein 7 0 0 0 0 0 100 2 75
L26 Hypothetic protein 7.5 0 0 0 0 0 100 4.5 66.7
L175 Hypothetic protein 3 0 0 0 0 0 0 4 0
Table 6 (cont.)
HUM Humidity
DRO Drought
SUBST Subtraction
Example 22. Classification of genes.
Among the genes that showed induction or repression signal in the macroarray according to example 21 , they were classified functionally; it is worth noting that the genes with values of zero when performing the subtraction were not classified, because this value indicates that those genes were not detected in control plants B73 and GM plants under drought stress conditions. According to the above, this functional classification found several genes associated with different functions in the cell, such as those associated or involved in abiotic stress
responses including drought (table 7), as is true of Dehydrines, belonging to a family group of LEA proteins (late embryogenesis abundant protein), which are produced in response to low temperatures, salinity and drought, for the protection of the membranes to stress damage (Allagulova et al., 2003; and Puhakainen et al., 2004), where its production is induced by abscisic acid (ABA). We also observed the protoplast intrinsic protein expression, which is a putative protein of aquaporin, and is directly directed to the use of water (Montalvo et al. 2007), and the kinase SPK-3 protein, which is involved in the induction of genes in response to ABA, to the activation of proteins at post-translational level (Yoon et al. 1997), and to the expression of LEA (late embryogenesis abundant protein) in group 4, which is involved in the protection of cellular components (Montalvo et al. 2007).
Table 7. Genes classified in functionality to abiotic stress response and suppression and induction levels in control and genetically modified according to the present invention
Genes classified as abiotic stress response showed different behaviors in B73 plants; about controls, these showed mainly a signal suppression of genes associated with drought stress response, contrary to the observed in GM plants according to the present invention (figure 25). We also observed signal intensities of genes that have functions in photosynthesis, which is defined as the series of reactions where the synthesis of ATP is produced by energized reactions by light and the fixation of C02 in the conversion of light energy to chemical energy; for instance we can note the importance of phosphoglucomutase, which is an enzyme responsible for converting glucose-1 -phosphate to glucose-6-phosphate, thus participating in glycogenolysis. Similarly to the presence of chloroplast proteins which is a cytoplasmic organelle responsible for performing photosynthesis in eukaryotic plant cells, algae, and some protists. We observed differences in gene expression at photosynthesis level, especially from GM to control plants, where the signals of these were of low expression (table 8 and figure 26). Significantly, we obtained a single value of signal intensity in control and genetically modified samples, classified in functional category of response to biotic stress (table 9). Thaumatin is a
protein that is involved in plant defense against pathogens known as PR proteins (pathogenesis-related) and osmotic stress response.
Table 8. Genes classified in functionality of photosynthesis and suppression and induction levels in control and genetically modified B73.
Table 9. Genes classified within the functionality of response to biotic stress and the suppression levels and induction in B73 control and genetically modified.
We should indicate that there was a gradual decrease in the expression signal relative to control and GM, where T4 showed signal suppression, indicating that the plant does not yet fully recognize the stress signals because of the genetic modification that occurs in its genome (figure 27).
Plants accumulate various compatible solutes, both for energy use and to protect stress response; that is the case of classified myoinositol gene associated with carbohydrate metabolism (table 10), and which a sugar responsible for maintaining the cell membrane and is synthesized from myo-inositol-1 -phosphate synthase, from which we could observe a signal suppression of this gene in GM maize plants according to the present invention (figure 28).
Table 10. Genes classified in functional response to biotic stress and suppression and induction levels in control and genetically modified B73
Table 1 1 and figure 29 show the signal intensity values obtained from unknown or hypothetical proteins in control and genetically modified B73; as we know, these proteins are unknown proteins whose function is not yet known and they are assigned a hypothetical functionality depending on their sequence and similarity with other already reported proteins. According to the present invention, we detected a total of 12 hypothetical proteins that are involved in the response to drought tolerance in maize, and that have been reported in other species of the plant kingdom.
Table 11. Genes classified as unknown and/or hypothetical proteins and indication of suppression and induction levels in control and genetically modified B73
According to the present invention, the expression assays on genes associated with the response to the drought show that the control and genetically modified plants display a different response. As reported, trehalose may be a multifunctional molecule, first as osmoprotectant; hence, the drought tolerance it provides to plants; and its signaling ability. Regarding the latter property, trehalose and its phosphorylated versions can induce a differential expression as reported by Iturriaga et al. (2009).
According to the present invention, the identification of the expression of some of the genes involved in drought tolerance shows the quantitative character of drought tolerance. So, observing that several genes are being induced supports the notion that not only trehalose and/or trehalose phosphate are sufficient to provide tolerance to abiotic stress. In this regard, we believe that possibly the effect of the accumulation of trehalose according to the present invention induces the expression or repression of genes that together produce a physiological state of tolerance to abiotic stress, in combination with an increase in photosynthetic rate. The ability to maintain autotrophy even under conditions of stress is a property of tolerant plants, and reflects that the plant still has a water status adequate to performing C02 fixation.
References.
1 Aguirre-Gomez, J.A., et.al 2000. A regional analysis of maize biological diversity in Southeastern Guanajuato, Mexico. Economic Botany. 54(1 ):60-72.
2 Allagulova ChR, Gimalov FR, Shakirova FM, Vakhitov VA. 2003. The plant dehydrins: structure and putative functions. Biochemistry (Mosc). 2003 Sep; 68(9): 945-51.
3 Aylor E.D., et.al. 2005. Some physical properties of teosinte (Zea mays subsp. parviglumis) pollen. J Exp Bot 56(419): 2401-2407.
4 Baltazar M.B, et.al. 2001 . Maize pollen biology, pollen drift and transgenes. In: Proc 56th Cornand Sorghum Seed Res Conf. Chicago.
5 Baltazar M.B., et.al. 2007. Assessment of potential impact of hybridization between teosinte (Zea spp.) and maize (Zea mays spp. mays) on dormancy characteristics of teosinte. Gene flow symposium at the North Center.
6 Baltazar M.B., et.al. 2005. Pollination between maize and teosinte: an important determinant of gene flow in Mexico. Theor Appl Genet. 1 10:519-526.
7 Bassetti P., et.al. 1993a. Emergence, elongation, and senescence of maize silks. Crop Sci.
33:271-275.
8 Bassetti P., et.al. 1993b. Senescence and receptivity of maize silks. Crop Sci. 33:275-278.
9 Benz, B.F. 2001 . Archaeological evidence of teosinte domestication from Guila Naquitz, Oaxaca. Proc. Natl. Acad. Sci. USA 98, 2104-2106.
10 Bellon M.R., et.al. 1994. Keepers of maize in Chiapas, Mexico. Econ. Bot. 48:196-209.
1 1 Castillo F (eds) ProcForum: gene flow among maize landraces, improved maize varieties, and teosinte: implications for transgenic maize. CIMMYT, Mexico City, pp 44-53
12 Castillo G.F., et.al. 1997. Research on gene flow between improved land races. In:
Serratos J.A., Willcox M.C., Castillo-Gonzalez F. (eds) Proc Forum: gene flow among maize landraces, improved maize varieties, and teosinte: implications for transgenic maize". CIMMYT, El Batan, Mexico, pp 67-72
13 Castro-Gil M. 1970. Frequencies of maize by teosinte crosses in a simulation of a natural association. Maize gen. Coop. Newsletter 44:21-24.
14 Cervantes M.J.E. 1998. Infiltracion genetica entre variedades locales e introducidas de maiz de sistema tradicional de Cuzalapa, Jalisco. PhD thesis, Colegio de Postgraduados, Montecillo-Texcoco, Edo. De Mexico, Mexico CIMMYT http://www.cimmyt.org/
15 Clegg, J.S. The physical properties and metabolic status of Artemia cysts at low water contents: "The water replacement hypothesis". In Membranes, Metabolism and Dry Organisms; Leopold, A.C., Ed.; Cornell University Press: Ithaca, NY, USA, 1985; pp. 169- 187.
16 De la Cruz L. 2007. Sistemas de incompatibilidad genetica en maiz y teosinte (Zea spp.) in Mexico. Tesis Doctoral. Universidad de Guadalajara, Zapopan, Jalisco. Enero 30, 2007.
17 Deng, M.Y., et.al. 1999. Molecular Characterization of Roundup Ready Corn Line NK603.
Technical Report MSL-16214, Monsanto Company, St. Louis, MO.
18 Di-Giovanni F., et.al. 1991. Factors affecting pollen dynamics and its importance to pollen contamination: a review. Can. J. For. Res. 21 :1 155-1 170.
19 Di-Giovanni F., et.al. 1995. The variability in settling velocities of some pollen and spores.
Grana 34:39-44.
20 Doebley, J. F. 1990. Molecular evidence and the evolution of maize. Econ. Bot. 44 (3 supplement): 6- 27.
21 Doebley J.F., et.al. 1980. Taxonomy of Zea (Gramineae). II. A subgeneric classification with key to taxa. Amer. J. Botany 67:982-993
22 Ellstrand N.C., et.al. 2007. Spontaneous hybridization between maize and teosinte. J. of Hered. 98(2):183-187.
23 Eroglu, A.; et.al. 2000. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol., 18, 163-167.
24 Eubanks M.W. 2001 . The mysterious origin of maize. Econ. Bot. 55:492-514.
25 Evans M.M.S., et.al. 2001 . Teosinte crossing barrierl , a locus governing hybridization of teosinte with maize. Theor. Appl. Genet. 103:259-265.
26 FAO http://www.fao.org/index_es.htm
27 Fink, L.A., et.al. 1983. Expression of bacterial genes in plant cells. Proc Nat Acad Sci U.S.A., 80(15), 4803-07.
28 Fuente AgBios http://www.agbios.com/.
29 Goggi, A.S., et.al. 2006. Statistical analysis of outcrossing between adjacent maize grain production fields, Field Crops research.
30 Gould, F.W. 1968. Grass Systematics. McGraw Hill, N.Y., USA.
31 Guadagnuolo R., et.al. 2006. Relative fitness of transgenic vs. non-transgenic maize x teosinte hybrids: a field evaluation. Ecol. Appl. 16(5): 1967-1974.
32 Guo, N.; et.al. 2000. Trehalose expression confers desiccation tolerance on human cells.
Nat. Biotech., 18, 168-171.
33 Iturriaga G, Suarez R, Nova-Franco B. 2009. Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci. 10:3793-810.
34 Jones, J.M., et.al. 1950. Effectiveness and distance of border rows in preventing outcrossing in corn. Oklahoma Agric. Exp. pollen Sta. Tech. Bull. No. T-38.
35 Jugenheimer, R.W. 1976. Maize Improvement, Seed Production, and Uses, John Wiley & Sons, Inc. New York, New York.
36 Kato Y.T.A. 1997. Review of introgression between maize and teosinte. In: Serratos JA, Willcox MC,
37 Kermicle J. 1997. Cross compatibility within the genus Zea. In: Serratos JA, Willcox MC, Castillo F (eds) Proc Forum: Gene flow among maize landraces, improved maize varieties, and teosinte: implications for transgenic maize. CIMMYT, Mexico City, pp 43
38 Kiesselbach, T.A. 1980. The structure and reproduction of corn. Re- print of: Research Bulletin No. 161. 1949. Agricultural Experiment Station, Lincoln, Nebraska. University of Nebraska Press, p. 93.
39 Kermicle J.. L. 1997. Tables and pathways, p. 306 in Mutants of Maize, edited by M. G.
NEUFFER, E. D. COE and S. R. WESSLER. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
40 Kiesselbach T.A. 1999. The structure and reproduction of corn, 50th anniversary. Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
41 Luna V.S., et.al. 2001. Maize pollen longevity and distance isolation requirements for effective pollen control. Crop Sci 41 :1551- 1557.
42 Mangelsdorf, P. C, et.al. 1981. The probable origin of annual teosintes. Bussey Inst., Harvard Univ. Publ. 10, 1- 69.
43 Martinez-Soriano J.P.R., et.al. 2000. Transgenic maize in Mexico: No need for Concern.
Sci. Vo. 278 (5457): 1399.
44 Mondrus-Engle, M. 1981. Tetraploid perennial teosinte seed dormancy and germination. J. of Range Manag. 34(1 ):59-61.
45 Montalvo-Hernandez L, Piedra-lbarra E, Gomez-Silva L, Lira-Carmona R, Acosta-Gallegos JA, Vazquez-Medrano J, Xoconostle-Cazares B, Ruiz-Medrano R. 2008. Differential accumulation of mRNAs in drought-tolerant and susceptible common bean cultivars in response to water deficit. New Phytol. 177:102-13.
46 Nida, D.L., et.al. 1996. Glyphosate-Tolerant Cotton: The Composition of the Cottonseed Is Equivalent to That of Conventional Cottonseed. J. Agric. Food Chem., 44:1967 -1974.
47 Ode J. T., et.al. 1985. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35s promoter. Nature 313, 810-12.
48 OECD. 2002. Consensus document on compositional considerations for new varieties of maize (Zea mays): key food and feed nutrients, anti-nutrients and secondary plant metabolites. Organisation for Economic Co-operation and Development, Environmental Health and Safety Publications. Paris, France. ENV/JM/MONO(2002)25
49 Puhakainen T, Hess MW, Makela P, Svensson J, Heino P, Palva ET .2004.
Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol Biol. 2004 Mar;54(5):743-53
50 Purseglove, J.W. (1972). Tropical Crops: Monocotyledons 1. Longman Group Limited., London. Raynor, G.S., Ogden, E.C. & Hayes, J.V. (1972). Dispersion and deposition of corn pollen from experimental sources. Agronomy Journal 64, 420-427.
51 Roser, B.; et.al. 1993. A sweeter way to fresh food. New Sci., 138, 25-28.
52 Rossi D. 2009. Maiz tolerante a sequia: el evento esperado. www.engormix.com/MA- agricultura/maiz/foros.
53 Watson, L., et.al. 1992. Grass Genera of the World: Descriptions, Illustrations, Identification, and Information Retrieval; including Synonyms, Morphology, Anatomy, Physiology, Phytochemistry, Cytology, Classification, Pathogens, World and Local Distribution, and References. Version:18th August
54 Watson, S.A. 1982. Maize: Amazing Maize. General Properties. In CRC Handbook of Processing and Utilization in Agriculture, Volume II: Part 1 Plant Products. Wolff, I.A. Ed.; CRC Press, Inc.: Boca Raton, FL; pp. 3-29.
Watson, S.A. 1987. Structure and composition. In Corn: Chemistry and Technology.
Watson, S.A., et.al. Eds.; American Association of Cereal Chemists, Inc.: St. Paul, MN; pp. 53-82.
Wilkes H.G. 1967. Teosinte: the closest relative of maize. Bussey Inst. Harvard Univ. 159 p.
Wilkes H.G. 1977. Hybridization of maize and teosinte in Mexico and Guatemala and the improvement of maize. Econ Bot 31 :254-293
WHO (World Health Organization). 1999. International Program on Chemical Safety (IPCS).
Yoon IS, Mori H, Kim JH, Kang BG, Imaseki H. 1997. VR-ACS6 is an auxin-inducible 1 - aminocyclopropane-1-carboxylate synthase gene in mungbean (Vigna radiata). Plant Cell Physiol. 1997 Mar;38(3):217-24.
1999. http://biodiversity.uno.edu/delta.