Flexible Geometries for Hand-held PET and
SPECT cameras
Irvmg N Wemberg, Valera Zavarzm, William Peter, Roberto Pam, JianChao Zeng, Members, IEEE, Pavel Stepanov, David Beyhn, Edward Anashkm, Giuseppe DeVincentes, Lee P. Adler
know a priori where the lesions are located. Because of this
Abstract — Minimally invasive surgery has spurred uncertainty, what may be an acceptable fϊeld-of-view for one development of portable nuclear medicine non-imaging patient's procedure may not be acceptable for the next detection products (i.e., probes). Although these probes function patient. As a result, no standard camera size can be specified well in simple anatomic regions (e.g., extremities), more for all surgeries Because useable camera fϊeld-of-view is complicated regions (e.g., head & neck, axilla) can present related to the overall physical size of the camera, a large surgeons with difficulties due to the presence of overlapping tissue activity. Conventional gamma cameras, with large camera field-of-view camera may be too heavy for surgeons to heads, are not ideal for intraoperative applications. Due to the manipulate, or may hinder access to lesions of interest in the bulk} camera size, PET and SPECT are difficult to implement close confines often encountered in surgery. Similarly, when in OR settings. Although small gamma cameras may be helpful the camera is moved from one area of interest to another, or when a lesion is already identified, surveillance of larger areas from one angular position to another, information from the (e.g., tor peritoneal evaluation) can be difficult due to limited earliest position is not integrated with information from iϊeld-of-view. Ideally, the information from small hand-held subsequent positions Because of this lack of integration gamma cameras would be integrated from multiple views taken between multiple views, it is difficult for the surgeon to at arbitrary positions and angles. ascertain the depth of a lesion
We present a method of ci eating an image with 3D Our vision was of a method that would record the position information from one or more portable hand-held gamma cameras held in arbitrary positions and angles near a and angulation of one oi more small field-of-view cameras, radioactive source [1]. The method takes advantage of recent and intelligently integrate the position and imaging data in developments in iterative reconstruction algorithms, computer oider to provide the user with a nearly real-time assessment speed, and position sensing devices. Corrections were of the three-dimensional source distribution Integrating implemented for inhomogeneous sampling in time and space. position and camera information for hand-held gamma cameras sol es several problems First, data are not discarded i INTRODUCTION when the surgeon moves the camera to different positions
INTRAOPER \ ΠVE visualization is a robust field, whose and angulahons Second, the depth of souices can be giovvlh is fueled by multiple factois Visualization of taiget calculated by integrating information ttom multiple views lesions and overlying tissues can leduce the time and Finally, the hand-held camera's field of view is determined mvasiveness of surgical procedures In addition to the by the position of the usei's hand, so that the camera's size is obvious cost savings derived from reducing time in the no longer the cπtical determinant of the field-of-view Just as operating room, additional benefits include reduced in a handheld non-imagmg probe, a small camera or set of complications, since experience has shown that complication cameras that can fit in close quarters can be moved by the rates are propoitional to the length of time for surgical user in order to accept information from a much large field- procedures [2,3] of-view For multiple cameias, there is no need for the
Gamma-ray probes have become the standard of care for cameras to be symmetiic m size For example, for prostate surgical procedures involving melanoma in the extremities and laparoscopic applications [7], we have experimented with (l e , legs and hands)[4] For some surgical sites involving a design incorporating an asymmetric pair of cameras, m complex anatomic regions (e g , head & neck, axilla, which a small camera could be inserted into body cavities, m abdomen) the lack of depth and size information prov ided by coincidence with a laiger external camera For prostate or simple non-imag g probes can reduce the efficacy of ovaπan examination, the external camera could be placed suigical guidance with gamma ray piobes [5] anteπoi to the urinary bladdei, while the mtracavitary
Some investigators have used small gamma cameras component could be placed endorectally or endovagmally, instead of non-imagmg ptobe detector heads This approach lespectively We piesent this paper to uitioduce the concept works well for a limited numbei of scenarios in which the of incorporating position and angular sensing into the data location of the lesion is already specified but the niaig s are acquisition paradigm foi nuclear medicine cameras, and not clear, as in osteoid osteoma [6] In cases wheie metastatic recognize that sophisticated algorithms and techniques to be disease is suspected, how ever, the surgeon generally does not
contributed by future workers may significantly improve performance of members of this class of flexible devices.
II. METHODS
A. Hardware
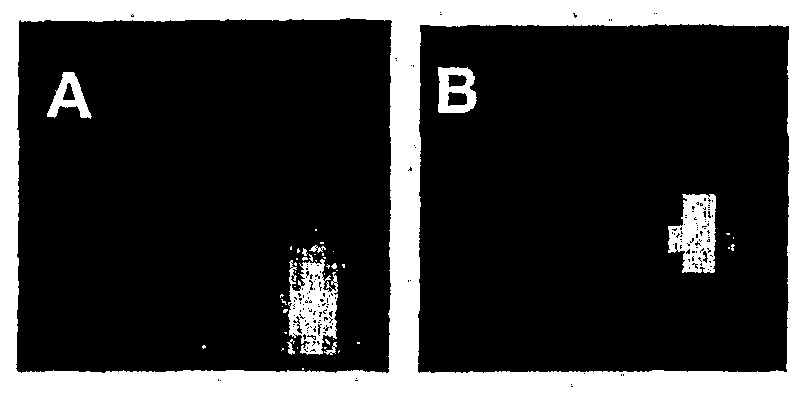
Several portable camera modules were built, using Hamamatsu R5900 C8 position-sensitive photomultipliers (PMTs) (Hamamatsu Photonics, Bridgewater NJ). For SPECT, the camera head included one R5900 PMT (Fig. 1, left) and weighed about 1 kg. The SPECT camera included housing and pinhole assembly fabricated using Kulite™ material, a tungsten slurry that is heated and compressed prior to machining. This material, which is has nearly identical stopping power to tungsten, is much easier to machine (Kulite™ Tungsten Products, H.C. Starck, East Rutherford NJ). A small pinhole size (2.5 mm diameter) was
selected, to provide good spatial resolution. A 7x7 GSO crystal array (3x3x3 mm
3) was affixed to the PMT face. For
PET, four R5900 PMTs were affixed to 18x18 arrays of
Fig 1. Cameras for handheld SPECT (left) and handheld PET (right), with 15x3x3 mm3 GSO crystals (Marubeni Specialty Chemicals, schematics shown below respective cameras. Each camera contains arrays White Plains NY), with no shielding. In the PET system, each of GSO crystals mounted or, light guides that are glued to position-sensitive camera head weighed less than 800 g. photomultipliers. In the case of handheld SPECT, a pinhole collimator
For both PET and SPECT, the detector heads were placed illuminates the crystal array. on handles to which Polhemus™ (Polhe us, Inc., Colchester VT) electromagnetic position receivers were affixed. For B. Algorithm Implementation those unfamiliar with this apparatus, the Polhemus™ system A graphic user interface was provided to the user, consists of an electromagnetic transmitter and multiple implemented in IDL (IDL Version 5.4, Research Systems, receivers. The Polhemus receiver is about one cm3 in size. Inc., Boulder CO), so that the position of the hand-held The Polhemus transmitter and detector are attached via cables camera was visible on the computer screen at all times. The to an electronics controller assembly. This assembly includes data stream containing position/angulation from the analog-to-digital conversion and power circuitry, and Polhemus sensor was integrated with the photomultiplier data coincidence triggers in the case of PET. The Polhemus stream and directed into short list-mode files that listed for circuitry interfaced with the computer via serial port, using every event both PMT and position data. These list mode ASCII commands that are interpreted by the controller files were read by the IDL routine approximately once a circuitry. Some custom configuration was required m order to second and backprojected in near real-time (approximately match the Polhemus™ Euler angle specification system with every five seconds) onto a set of virtual planes. the standard "Goldstein" type angles used in most Through a straightforward formalism, it was possible to reconstruction programs [8]. The camera hardware and treat data from both handheld PET and SPECT systems in a configuration of the Polhemus™ receiver and transmitter are manner similar to the method we have used for positron shown in Fig. 1 for the handheld SPECT and PET systems. emission mammography [9]. For rapid backprojection a tomosynthesis display method was employed, first described Although the Polhemus™ system is susceptible to distortion in 1976 [10] and later adapted for positron emission from metal objects, correction software is available from the mammography [9]. The depth information presented by this manufacturer to reduce such artifacts. display method is similar to what radiologists expect of non- computed tomographic images (e.g., as in intravenous urograms), in which a structure appears sharpest in a single plane and is blurred in other planes. A reconstruction method is described later in this section.
The sequence of data and image processing can be described as follows for handheld PET: Each event triggered by coincidence from the two camera modules is assigned to particular crystals in each detector array ("event origins"). Because the camera modules are handheld, the locations of the two event origins are dependent on the position and angulatiυn of the receiver. Since each detector array and
Polhemus receiver are on a single rigid body, the Euler angle For SPECT with a single handheld pinhole camera, each formalism allows transformation from the rotating and event is assigned to a particular crystal in the detector array translating camera frames of reference to the stationary frame ("event origin"). Both the location of the event origin and the of reference [8]. The computer program utilizes the angular location of the center of pinhole are dependent on the and position information in the list mode files to calculate the position and angulation of the receiver. Since the pinhole, location of the event origins in the stationary frame of detector array, and Polhemus receiver are all on a single rigid reference (Fig. 2, left). A virtual ray is then generated body, the Euler angle formalism allows transformation from between the event locations using the two-point formula for a the rotating and translating camera frame of reference to the line. The virtual ray is allowed to project onto multiple stationary frame of reference [8]. The computer program virtual planes in the volume of interest specified by the user. utilizes the angular and position information in the list mode At every point on each virtual plane that the virtual ray files to calculate the location of the event origin and center of intersects, the virtual plane's pixel value is incremented. the pinhole in the stationary frame of reference. A virtual ray is then generated arising from this event location, whose direction is determined using the two-point formula for a line. These two points (in the stationary reference frame) include the event location, and the center of the pinhole. The virtual ray is allowed to project onto multiple virtual planes. At every point on each virtual plane that the virtual ray intersects, the virtual plane's pixel value is incremented.
In the SPECT case, motion of the handheld camera causes a blurring in each virtual plane that does not actually contain the source. In the virtual plane whose depth corresponds to the actual depth of the source, rays still converge to a point. As expected for a tomographic system, backprojecting from a moving pinhole camera has the salutary effect of distinguishing overlapping sources from one another (Figure 3). In the PET case, motion of the handheld camera components is not necessary to achieve focusing in the virtual plane whose depth corresponds to the source's actual depth. Because hand-held cameras by definition have no predetermined orbit, the efficiency of data collection must be calculated for each actual position and angle in the orbit. Due to constraints on processing times, it is not practical to perform a reconstruction for all possible orbits and for all possible source volumes.
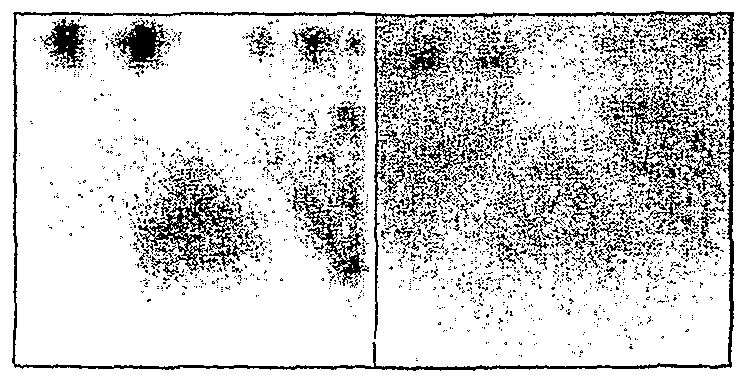
Fig 3. Simulation results for handheld SPECT: Reducing the influence of overlapping sources by backprojecting from moving pinhole camera.
(A) Simulation conditions in which three coplanar sources (a, b, c) are
placed in plane X = 100, and one source (d) placed in plane X = 0.
Fig 2. Graphical User Interfaces showing location of camera heads in (B) Image as seen by pinhole camera when no camera motion occurs. The handheld single-head SPECT (on top), and handheld dual-camera PET sources appear inverted because of pinhole geometry. All four sources (a, b, system (on bottom). Backprojected rays are drawn in the stationary frame c, d) contribute to the image. Because of overlap from source d, images of of reference (between dual detector heads in the PET system, and emanating sources a and b cannot be individually resolved. from the pinhole camera in the SPECT case). (C) Backprojected image at plane X =100 obtained with simulation of moving pinhole camera. Backpi'ojeciion removes the image of source d (at X=0) from the images of coplanar sources a, b, c. As expected for a tomographic system, the influence of overlapping sources is reduced, so that sources a and b can be resolved as being separate.
To make the problem of reconstruction tractable, we took III. EXPERIMENTAL RESULTS the approach of prescribing a finite selected reconstruction Images obtained with the hand-held SPECT and PET volume. Because we were familiar with the reconstruction cameras are shown in Figs. 5-7. problem for the limited angle set encountered in positron emission mammography (PEM), we attempted to cast the problem into a PEM-like geometry, in which virtual planes on opposite sides of the reconstruction volume were selected to bound the reconstruction volume. Then the coordinates of intersection of each ray onto each of these boundary virtual planes can be input to the PEM reconstruction algorithm as if there were actual detectors on the virtual boundary planes. This formalism was employed for rays projected from a
handheld SPECT camera, as well as for rays projected Fig 5. Experimental results for conventional Siemens Orbiter gamma between the handheld PET camera heads (Figure 4). camera with parallel hole collimator (left) vs. handheld SPECT (right). Phantom consists of a one-cm hot spot in a warm surround [12].
In the hand-held PET case (Fig. 6), two Na-22 point sources were separated with a 3-mm thick disk. Due to the improved spatial resolution of the handheld camera, the two points were readily resolvable as being separate, while a 10- minute acquisition in a whole-body PET scanner failed to resolve the points.
Fig 4. Formalism for prescribing reconstruction volume in the stationary frame of reference. From the point of view of reconstruction, backprojected
rays are considered to have arisen from "virtual detectors" tocated on the boundaries of the prescribed reconstruction volume. In both the PET case Fig 6. Experimental image of one microCurie Na-22 point sources as (left) and the pinhole SPECT case (right), the camera heads lay outside the viewed by handheld PET (left) vs. conventional whole-body PET scanner prescribed reconstruction volume. (ADAC C-PET™) (right), each for four minute acquisition time. Point sources in air, separated by 3 mm metal disk (a U.S. quarter), are separately
A first-order correction was implemented for spatial and resolvable only with high-resolution handheld PET detectors. temporal sampling inhomogeneity in the handheld scanner orbits by calculating the efficiency of the acquisition orbit in collecting counts from a uniformly distributed source filling the specified reconstruction volume of interest. The implemented efficiency correction consisted of backprojecting (onto virtual planes within the selected reconstruction volume) all possible events that could have been detected on the detector plane for the time that the handheld detector was in each particular position. The actual
event backprojection was then divided by this efficiency Fig 7. Experimental image of resolution phantom as viewed by handheld backprojection to get an efficiency-corrected backprojection PET in five-minute acquisition (left) vs. conventional whole-body PET data set. Reconstruction for the handheld systems was scanner after 30-minute acquisition (right). Hot spots vary in size from 1 to 3 mm in diameter, with 5:1 hot spot to background ratio. Whole-body accomplished with an iterative limited-angle reconstruction image can only resolve 3 mm diameter hoi spots, while handheld PET algorithm reconstruction algorithm (based on the Image resolved hot spots 2 mm in size or larger. Space Reconstruction Method) previously employed for positron emission mammography [1 1].
V. REFERENCES
[I] Wemberg, Irving N. United States Patent application number
IV. CONCLUSIONS 09/833,1 10
It is possible to incorporate position sensing in the [2] Longer duration of caidiopulmonary bypass is associated with greater numbers of cerebral microemboli. Brown WR, Moody DM, Challa backprojection and reconstruction process for a hand-held VR, Stump DA, Hammon JW. Stroke 2000 Mar;31(3):707-13. small gamma camera or PET scanner. The same formalism [3] Risk factors for surgical wound infections in patients undergoing head could easily be applied to distributed gamma camera sensors. and neck oncologic surgery. Barry B, Lucet JC, Kosmann MJ, Gehanno P. Acta Otorhinolaryngol Belg l999;53(3):241-4. The cameras need not be symmetric in size, and we have [4] Sentinel node localisation: A new prospective in the treatment of demonstrated how small intracavitary (e.g., endorectal) nodal melanoma metastases. Gennaπ R, Stoldt HS, Bartolomei M et components can be incorporated into PET systems using al. Int J Oncol 1999 Jul; 15(l):25-32.
[5] Use of the sentinel lymph node to determine metastases of position sensors [7] (Fig. 8). These flexible systems can also gastrointestinal malignancies, a word of caution. Chin PL, Medeiros J, employ biopsy needles using position sensors, to create Schwarz RE. J Surg Oncol 1999 Aug;71(4):239-42. virtual displays for biopsy and/or needle-wire guidance [12]. [6] Intraoperative use of the mobile gamma camera in localizing and excising osteoid αsteomas of the spine. Osebold WR, Lester EL, Hurley JH, Vincent RL. Spine 1993 Oct l;18(13).1816-28.
We present this paper on flexible geometries for hand-held [7] Design of Biopsy-Enabled Intracavitary PET Scanner for Detection cameras as a work-in-progress, and as such we hope that and Delineation of Prostate Cancer. Wemberg IN, Adler LP, Zavarzin V, Stepanδv P, Peter W, Zeng JC, Opell B Presented at Second future workers in the imaging field would contribute ideas Internationa! Innovative Solutions for Prostate Cancer Care: Image- that could significantly improve on the performance of handguided Minimally Invasive Diagnosis and Treatment, San Diego, Feb. held cameras. We envision, for example, algorithmic 2001 feedback which would recommend to human users particular [8] Classical Mechanics, H. Goldstein. Addison-Wesley Publishing Company orbits for real-time imaging (i.e., human-centered robotics). [9] Feasibility Study for Positron Emission Mammography. Thompson Additionally, list-mode reconstruction methods could GJ, Murthy K, Wemberg IN, et al Med Phys 1994, 21.529-538. potentially reduce computational overhead [13]. Other [10] Performance Parameters of a Positron Imaging Camera. Muehllenner G, Buchin MP, Dudek JH. IEEE Trans. Nucl. Sci 1976. 23*528-537 challenges to future researchers include attenuation [II] Monte Carlo-based Implementation of the ML-EM Algorithm for 3-D correction, which could perhaps be implemented through PET Reconstruction, Worstell W, Kudrolh H, Zawarzin V. point sources attached to one of the camera heads. Finally, Proceedings IEEE Nucl. Sci. Sy p 1998.
[12] Simulated Performance of Radiotracer-Guided Breast Biopsy Kim SP, the current crop of commercially available position sensing Khalkhali I, Diggles LE, Vargas HI, Weinberg IN Radiology 2000 devices are not ideal, and software/hardware corrections to 217 P 706 the outputs of these devices may need to be improved in [13] Entwicklung ernes iterativen 3D Rekonstruktionsverfahrens fur die Kontrolle der Tumorbehandlung mit Scheπonen mittles der order to be widely accepted in the operating room Positronen-Emissions-Tomographie. Lauckner K Ph.D Thesis environment. Wissenschafthch-Technische Beπchte FZR-264, June 1 99.
[14] Technical improvements in F-18 FDG PET imaging of the abdomen and pelvis Leisure GP, Vesselle HJ, Faulhaber PF, O'Donnell JK, Adler LP, Miraldi F J Nucl Med Technol 25 1 15-1 19, 1997
Fig 8. Conceptual drawing of endocavitary PET scanner foi prostate [1 J Stationary camera head external to body (#1 ) forms a detector pan with handheld endocavitary camera (#5) on opposite side of prostate (#4) The endocavitary head can be atfixed to an ultrasound camera, and can also admit a needle foi biopsy oi energy delivery for minimally invasive interventions (#6,87) To reduce the singles rate, the urinary bladder (#3) can be diained via a catheter, as has been done for whole-body PET [ 14]








