WO2002013826A1 - Anti-cancer composition composed of anti-cancer and anti-malarial drugs - Google Patents
Anti-cancer composition composed of anti-cancer and anti-malarial drugs Download PDFInfo
- Publication number
- WO2002013826A1 WO2002013826A1 PCT/KR2001/001314 KR0101314W WO0213826A1 WO 2002013826 A1 WO2002013826 A1 WO 2002013826A1 KR 0101314 W KR0101314 W KR 0101314W WO 0213826 A1 WO0213826 A1 WO 0213826A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- drugs
- anticancer
- drug
- cancer
- antimalarial
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/243—Platinum; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
- A61K31/704—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin attached to a condensed carbocyclic ring system, e.g. sennosides, thiocolchicosides, escin, daunorubicin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- the present invention relates to a complex composition of an anticancer drug in combination with an antimalarial drug for reducing a minimum IC 50 of the anticancer drug and inhibiting development of drug resistance in cancer cells caused by exposure to the anticancer drug, thereby enhancing the effect of the anticancer drug.
- the present invention relates to a complex composition of an anticancer drug in combination with an antimalarial drug for reducing a minimum IC 50 of the anticancer drug and inhibiting development of drug resistance in cancer cells caused by exposure to the anticancer drug, thereby enhancing the effect of the anticancer drugs;
- the anticancer drug is selected from the group consisting of doxorubicin, cisplatin, and the like and the antimalarial drug is selected from the group consisting of hydroxychloroquine, chloroquine, primaquine and the like.
- the efficacy of the anticancer drugs can be increased by approximately 3 times for breast cancer, approximately 10 times for gastric cancer, approximately 10 times for colon cancer and approximately 10 times for sarcoma, as compared with the treatment with of the anticancer drugs only.
- MDR Multidrug resistance
- Anticancer drugs which have been found to be associated with the multidrug resistance include anthracycline family drugs such as adriamycin and daunorubicin; vinka alkaloid family drugs such as vincristine and vinblastin; epipodophllotoxin family drugs such as etoposide; and others such as actinomycin-D and taxol . Also, it was found that these drugs induce cross-resistances with each other.
- Such anticancer drugs are commonly characterized in that they are hydrophobic materials having a molecular weight of 300 to 900; that they comprise complicated ring structures and have a nitrogen group of a positive charge; and that they are passively diffused into the cells.
- Gros et al examined the cDNA base sequence of a gene for p-glycoprotein, and made the first identification of MDR1 (Multidrug Resistance 1) gene, which is associated with multidrug resistance.
- the p-glycoprotein includes 1280 amino acids and has a structure similar to those of transport proteins such as hemolysin B, leukotoxin, histidine, and the like. It was also found that transporting proteins having structures similar to that of the p-glycoprotein exist in bacteria and yeast. Thus, it is noted that the p-glycoprotein can be found in normal cells of the small and large intestines, adrenal glands, kidneys, liver, etc. as well as tumor cells and is a variety of transporting proteins to play a general role for transporting intracellular cytotoxin out of cells.
- the p-glycoprotein mainly distribute in the biliary tract in the liver, luminar surface in proximal tubules of kidneys, columnar cells in luminar mucous membrane of small and large intestines, and adrenal cortex and medulla. Also, it was shown that levels of mdrl mR A are high at diagnosis in some cancers including colon cancer, kidney cancer, liver cancer, chronic leukemia, adrenal carcinoma and non- small cell cancer, which indicates endogenous multidrug resistance (see, Fojo AT, Ueda K, Slamon DJ, Poplack DG and Gottesman MM, Pastan I: Expression of a multidrug resistance gene in human tumors and tissues, Proc. Natl. Acad. Sci.
- the overexpression of mdrl mRNA and p-glycoprotein are considered to be a major cause of failure of the treatment. These overexpressions are sometimes referred to as indicators or factors forecasting the efficacy of the treatment and prognosis.
- Tsuro et al disclosed a use of a calcium channel blocker, for example, verapamil, based on the fact that the cancer cells showing multidrug resistance contains a large amount of calcium in plasmalemma and cytoplasm, compared to sensitive cancer cells.
- Hydroxychloroquine is one of well known antimalarial drugs and is sometimes used as an anti- inflammatory agent for various rheumatic diseases. It is known that antimalarial drugs including hydroxychloroquine exhibit pharmacological effect by increasing the pH of intracellular organelles including the lysosome, endosome and the trans -Golgi network (see, Fox RI, Mechanism of Action of Hydroxychloroquine as an Antirheumatic Drug, Seminars in Arthritis & Rheumatism, 23 (2 Suppl. 1):82-91, 1993; Fox R, Antimalarial drugs: Possible Mechanisms of Action in Autoimmune Disease and Prospects for Drug Development, Lupus, 5 Suppl. 1.S4-10, 1996) . Also, it was reported that alkalinization of intracellular organelles affects the secretion of proteins synthesized intracellularly and also inhibits the synthesis of DNA and RNA.
- the present inventors studied the effectiveness of combined administration of an anticancer drug with an antimalarial drug to inhibit the development of drug resistance in cancer cells.
- the used anticancer drug includes doxorubicin and cisplatin.
- the used antimalarial drug includes hydroxychloroquine, chloroquine and primaquine.
- Fig. 1 is a graph depicting the changes in cytotoxicity of doxorubicin (ADR) and cisplatin (DDP) when combined with hydroxychloroquine at concentrations of 15 and 30 ⁇ g/ml in colon and gastric cancer cell lines, respectively (The X axis represents administered concentrations of the anticancer drug. The Y axis represents cell viability) ; and
- Fig. 2 is a graph depicting the changes in cytotoxicity of ADR and DDP when combined with either chloroquine at concentrations of 20 ⁇ M (10.318 ⁇ g/ml) and 40 ⁇ M (20.636 ⁇ g/ml), or primaquine at concentrations of 1.5 ⁇ M (0.683 ⁇ g/ml) and 3 ⁇ M (1.366 ⁇ g/ml) in breast cancer, gastric cancer and fibrosarcoma cell lines, respectively (The X axis represents administered concentrations of anticancer drug. The Y axis represents cell viability) .
- Colon cancer cell lines of HT-29 (ATCC HTB38, human colonic adenocarcinoma, moderately well differentiated grade II) and HCT-15 (ATCC CCL225, human colonic adenocarcinoma) , gastric cancer cell lines of KHH (YCC-2, human gastric adenocarcinoma), PHB (YCC-3, human gastric adenocarcinoma), KMB (YCC-7, human gastric adenocarcinoma) and AGS (ATCC CRL 1739, human gastric adenocarcinoma) , a fibrosarcoma cell line of HT 1080 (ATCC CCL 121, human fibrosarcoma) , and breast cancer cell lines of SK-BR-3 (ATCC HTB 30, human breast adenocarcinoma, malignant pleural effusion) are maintained in RPMI 1640 medium (Gibco, U.S.
- FCS fetal calf serum
- the anticancer drugs used in the experiments are adriamycin (ADR, generic name doxorubicin) , supplied by Farmitalia Carlo Erba Ltd. (Italy) , and diaminodichloro platinu (DDP, generic name cisplatin), supplied by Pharmachemie B.V. (Holland) .
- ADR generic name doxorubicin
- DDP diaminodichloro platinu
- the antimalarial drugs used in the experiments were hydroxychloroquine, chloroquine, and primaquine.
- the anticancer drugs are administered in combination with the antimalarial drugs varying proportions and are examined for cytotoxicity according to MTT assay using 3- (4 , 5-dimethylthiazol-2-yl) -2 , 5- diphenyl-1-butene (Sigma, U.S.A.). Particularly, ADR is used at concentrations of 10, 1, 0.1 and 0.01 ⁇ g/ml by cascade dilutions in each column on a microplate while DDP is assayed at 50, 5, 0.5, 0.05 ⁇ g/ml.
- hydroxychloroquine is examined at concentrations of 15 and 30 ⁇ g/ml, chloroquine at 10.3 and 20.6 ⁇ g/ml, and premaquine at 0.68 and 1.36 ⁇ g/ml. Cytotoxicity (anticancer effect) test by MTT assay
- the principle of the MTT assay is based on the phenomenon that succinate dehydrogenase, an mitochondrial enzyme located at cytochrome b and c of a living cell, cleaves tetrazolium ring of 3- [4,5- dimethylthiazol-2-yl] -2, 5-diphenyltetrazolium bromide (MTT) , whereby the yellow color of the MTT salt changes to the purple color of formazan, a reduced product. That is, the MTT assay cannot be observed in media of dead cells or tissues, but can be observed selectively only in the viable cells. The color change is measured by counting living cells by means of a spectrophotometer .
- the MTT assay is now one of the in vitro test methods for sensitivity of human tumor cells to anticancer drugs which are now recommended by the National Cancer Institute in U.S.A., due to its excellent reproducibility on repeated experiments.
- the MTT assay is performed as described by Carmichael et al .
- the standard growth curve of each cultured cancer cell line is generated to determine the exponential growth period of each cell line.
- each exponentially dividing cell line is treated with 0.25% trypsin-EDTA, suspended in a single cell population and washed three times with RPMI 1640 medium containing 10% FCS .
- Cells in each culture are counted by staining with Trypan blue (Gibco, U.S.A.) .
- 180 ⁇ l of cultures at the exponential phase are aliquoted on a 96 well plate (Costar, U.S.A), followed by an incubation at 37°C under 5% C0 2 .
- anticancer drugs at various concentrations are added either alone or in combinations with antimalarial drugs, each dissolved in saline at a volume of 20 ⁇ l, followed by a further 4 days incubation. 50 ⁇ l of MTT (2 mg/ l stock) is added to the wells and further incubated for 4 hours. Control cultures include equivalent amounts of saline instead of antimalarial drug.
- the plate is centrifuged at 450 g for 10 minutes. Supernatants are carefully removed from the well plate with the aid of digital multichannel pipette (Flow Titertck, Finland) to leave a 30 ⁇ l of culture in each well.
- each IC 50 of doxorubicin (ADR) and cisplatin (DDP) was measured when combined with hydroxychloroquine (HCQ) at concentrations of 15 ⁇ g/ml and 30 ⁇ g/ml in cancer cell lines.
- HCQ hydroxychloroquine
- the combinations of ADR with HCQ showed about 10 fold increased anticancer effects in colon cancer cell lines, HT-29 and HCT-15, about 3 to 10 fold in gastric cancer cell lines, YCC-2, YCC-3 and YCC-7. Also, the combinations of DDP with HCQ showed anticancer effects increased 2 to 5 fold in colon cancer cell lines, about 4 to 15 fold in gastric cancer cell lines, compared with the treatment with anticancer drug only.
- each IC 50 of doxorubicin (ADR) and cisplatin (DDP) was measured when combined with chloroquine (CQ) at concentrations of 20 ⁇ M (10.318 ⁇ g/ml) and 40 ⁇ M (20.636 ⁇ g/ml) in cancer cell lines.
- CQ chloroquine
- the combinations of ADR with CQ showed anticancer effects improved by 1.4 to 3 fold in a breast cancer cell line, SK-Br-3, about 6 to 10 fold in gastric cancer cell lines, AGS and YCC-7, and about 4 to 10 fold in fibrosarcoma cell line, HT1080.
- the combinations of DDP with CQ showed anticancer effects about 2 to 2.3 fold in the breast cancer cell line, about 1 to 5 fold in the gastric cancer cell lines, and about 2.3 to 12 fold in the fibrosarcoma cell line, compared with the treatment with anticancer drug only.
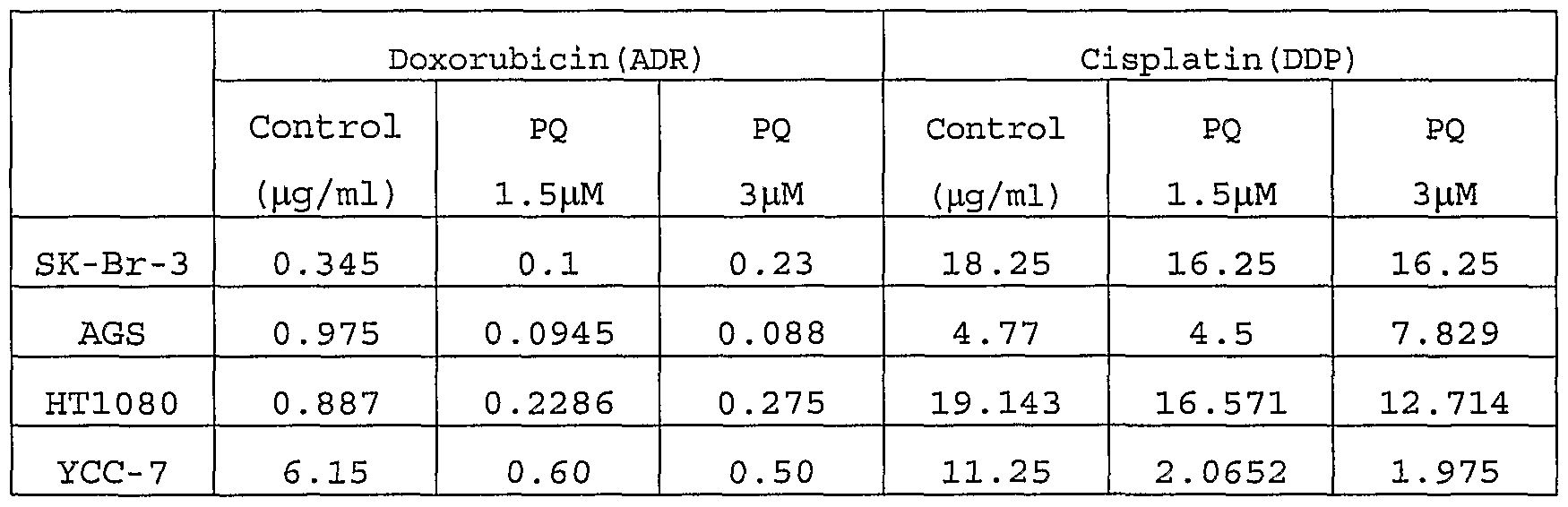
- each IC 50 of doxorubicin (ADR) and cisplatin (DDP) was measured when combined with primaquine (PQ) at concentrations of 1.5 ⁇ M (0.683 ⁇ g/ml) and 3 ⁇ M (1.366 ⁇ g/ml) in cancer cell lines.
- PQ primaquine
- the combinations of ADR with PQ showed anticancer effects increased by about 3 fold in a breast cancer cell line, SK-Br-3, about 10 fold in gastric cancer cell lines, AGS and YCC-7, and about 3 to 4 fold in fibrosarcoma cell line, HT1080.
- the combinations of DDP with PQ showed anticancer effects improved by about 1.2 fold in the breast cancer cell line, about 1 to 6 fold in the gastric cancer cell lines, and about 1.3 fold in the fibrosarcoma cell line, compared with the treatment with anticancer drug only.
- Administration of the antimalarial drug may be performed by either oral or parenteral routes in accordance with the anticancer drugs.
- hydroxychloroquine, chloroquine and primaquine are administered to humans at a dose of 0.1 to 500 mg/kg, 0.1 to 700 mg/kg, and 0.1 to 800 mg/kg, respectively, together with an anticancer drug in the amount which has been conventionally used for chemotherapy by practitioners skilled in the art. More preferably, 10 to 100 mg/kg for hydroxychloroquine, 10 to 300mg/kg for chloroquine, and 50 to 500 mg/kg for primaquine are administered.
- the present invention provides a complex composition
- the composition of the invention lowers the IC 50 of anticancer drug and inhibits the development of drug resistance in cancer cells.
- the composition is therefore capable of enhancing the effectiveness of chemotherapy about 3 fold in breast cancer, about 10 fold in gastric cancer, colon cancer, and sarcoma, respectively, compared with the treatment with anticancer drug only.
Abstract
Description
Claims
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2001275821A AU2001275821A1 (en) | 2000-08-02 | 2001-08-02 | Anti-cancer composition composed of anti-cancer and anti-malarial drugs |
| EP01953364A EP1313475A4 (en) | 2000-08-02 | 2001-08-02 | Anti-cancer composition composed of anti-cancer and anti-malarial drugs |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR10-2000-0044851A KR100390332B1 (en) | 2000-08-02 | 2000-08-02 | anti-cancer composition composed of anti-cancer and anti-malarial drugs |
| KR2000/44851 | 2000-08-02 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2002013826A1 true WO2002013826A1 (en) | 2002-02-21 |
| WO2002013826A9 WO2002013826A9 (en) | 2003-11-13 |
Family
ID=19681440
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2001/001314 WO2002013826A1 (en) | 2000-08-02 | 2001-08-02 | Anti-cancer composition composed of anti-cancer and anti-malarial drugs |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP1313475A4 (en) |
| KR (1) | KR100390332B1 (en) |
| AU (1) | AU2001275821A1 (en) |
| WO (1) | WO2002013826A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7108992B2 (en) | 2002-11-27 | 2006-09-19 | St. Jude Children's Research Hospital | ATM kinase compositions and methods |
| US7160692B2 (en) | 2002-11-27 | 2007-01-09 | St. Jude Children's Research Hospital | ATM kinase compositions and methods |
| WO2008038291A1 (en) * | 2006-09-27 | 2008-04-03 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Combination of liposomal anti-cancer drugs and lysosome/endosome ph increasing agents for therapy |
| WO2011076547A1 (en) | 2009-12-23 | 2011-06-30 | Sigma-Tau Industrie Farmaceutiche Riunite S.P.A. | Anticancer combination of artemisinin-based drugs and other chemotherapeutic agents |
| US20120095045A1 (en) * | 2009-06-24 | 2012-04-19 | Oh-Young Yeo | Injectable anticancer composition for local administration containing hydroxychloroquine |
| CN102481252A (en) * | 2009-06-23 | 2012-05-30 | 吕旿荣 | Injectable composition containing hydroxychloroquine for local administration for treating hemorrhoids |
| WO2014052550A1 (en) * | 2012-09-27 | 2014-04-03 | Thomas Jefferson University | Use of parp inhibitors to treat breast cancer |
| AU2012201742B2 (en) * | 2005-01-19 | 2015-05-14 | The Trustees Of The University Of Pennsylvania | Regulation of autophagy and cell survival |
| WO2019138203A1 (en) * | 2018-01-15 | 2019-07-18 | Inoviem Scientific | Composition comprising hydroxychloroquine and therapeutic use |
| WO2021105761A1 (en) * | 2019-11-29 | 2021-06-03 | Institut National De La Santé Et De La Recherche Médicale (Inserm) | Compositions and their uses for treating cancers |
| RU2764175C1 (en) * | 2018-06-19 | 2022-01-14 | Армасьютика Инк. | Bifunctional compositions for cancer treatment |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101068202B1 (en) * | 2009-10-27 | 2011-09-28 | 조재용 | Anti-cancer agent composition for stomach cancer comprising vorinostat |
| KR101769666B1 (en) | 2015-04-13 | 2017-08-21 | 영남대학교 산학협력단 | Anti-cancer pharmaceutical composition comprising doxorubicin and irinotecan and manufacturing method thereof |
| KR101698003B1 (en) | 2016-06-20 | 2017-01-19 | 여오영 | Injectable composition for topical administration for cancer treatment that comprising a quinine salt suspension |
-
2000
- 2000-08-02 KR KR10-2000-0044851A patent/KR100390332B1/en not_active IP Right Cessation
-
2001
- 2001-08-02 EP EP01953364A patent/EP1313475A4/en not_active Ceased
- 2001-08-02 AU AU2001275821A patent/AU2001275821A1/en not_active Abandoned
- 2001-08-02 WO PCT/KR2001/001314 patent/WO2002013826A1/en active Application Filing
Non-Patent Citations (7)
| Title |
|---|
| BECK W.T. ET AL.: "Effects of indole alkaloids on multidrug resistance and labeling of P-glycoprotein by a photoaffinity nalog of vinblastine", BIOCHEM. BIOPHYS. RES. COMMUN., vol. 153, no. 3, 1988, pages 959 - 966, XP001194406 * |
| HURWITZ S.J. ET AL.: "Vesicular anthracycline accumulation in doxorubicin-selected U-937 cells: participation of lysosomes", BLOOD, vol. 89, no. 10, 1997, pages 3745 - 3754, XP002981111 * |
| QUARLESS S.A.: "Chloroquine enhances the cytotoxicity of cis-diamminedichloroplatinum II", CANCER BIOCHEM. BIOPHYS., vol. 10, no. 1, 1988, pages 11 - 15, XP009032938 * |
| See also references of EP1313475A4 * |
| VEZMAR M., GEORGES E.: "Reversal of MRP-Mediated doxorubicin resistance with quinoline-based drugs", BIOCHEM. PHARMACOL., vol. 59, no. 10, May 2000 (2000-05-01), pages 1245 - 1252, XP002981110 * |
| ZAMORA J.M. ET AL.: "Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells", MOL. PHARMACOL., vol. 33, no. 4, 1988, pages 454 - 462, XP000569171 * |
| ZAMORA J.M., BECK W.T.: "Chloroquine enhancement of anticancer drug cytotoxicity in multiple drug resistant human leukemic cells", BIOCHE. PHARMACOL., vol. 35, no. 23, 1986, pages 4303 - 4310, XP002981112 * |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7160692B2 (en) | 2002-11-27 | 2007-01-09 | St. Jude Children's Research Hospital | ATM kinase compositions and methods |
| US7279290B2 (en) | 2002-11-27 | 2007-10-09 | St. Jude Children's Research Hospital | ATM kinase compositions and methods |
| US7108992B2 (en) | 2002-11-27 | 2006-09-19 | St. Jude Children's Research Hospital | ATM kinase compositions and methods |
| AU2012201742B2 (en) * | 2005-01-19 | 2015-05-14 | The Trustees Of The University Of Pennsylvania | Regulation of autophagy and cell survival |
| WO2008038291A1 (en) * | 2006-09-27 | 2008-04-03 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Combination of liposomal anti-cancer drugs and lysosome/endosome ph increasing agents for therapy |
| CN102481252A (en) * | 2009-06-23 | 2012-05-30 | 吕旿荣 | Injectable composition containing hydroxychloroquine for local administration for treating hemorrhoids |
| CN102481253B (en) * | 2009-06-24 | 2014-06-25 | 吕旿荣 | Injectable composition containing hydroxychloroquine for local administration for treating cancer |
| CN102481253A (en) * | 2009-06-24 | 2012-05-30 | 吕旿荣 | Injectable composition containing hydroxychloroquine for local administration for treating cancer |
| EP2465494A2 (en) * | 2009-06-24 | 2012-06-20 | Oh-Young Yeo | Injectable composition containing hydroxychloroquine for local administration for treating cancer |
| EP2465494A4 (en) * | 2009-06-24 | 2013-02-27 | Oh-Young Yeo | Injectable composition containing hydroxychloroquine for local administration for treating cancer |
| US8506973B2 (en) * | 2009-06-24 | 2013-08-13 | Oh-Young Yeo | Injectable anticancer composition for local administration containing hydroxychloroquine |
| US20120095045A1 (en) * | 2009-06-24 | 2012-04-19 | Oh-Young Yeo | Injectable anticancer composition for local administration containing hydroxychloroquine |
| US9023861B2 (en) | 2009-12-23 | 2015-05-05 | Sigma-Tau Industrie Farmaceutiche Riunite, S.P.A. | Anticancer combination of artemisinin-based drugs and other chemotherapeutic agents |
| WO2011076547A1 (en) | 2009-12-23 | 2011-06-30 | Sigma-Tau Industrie Farmaceutiche Riunite S.P.A. | Anticancer combination of artemisinin-based drugs and other chemotherapeutic agents |
| WO2014052550A1 (en) * | 2012-09-27 | 2014-04-03 | Thomas Jefferson University | Use of parp inhibitors to treat breast cancer |
| WO2019138203A1 (en) * | 2018-01-15 | 2019-07-18 | Inoviem Scientific | Composition comprising hydroxychloroquine and therapeutic use |
| FR3076711A1 (en) * | 2018-01-15 | 2019-07-19 | Inoviem Scientific | COMPOSITION COMPRISING HYDROXYCHLOROQUIN AND THERAPEUTIC USE |
| RU2764175C1 (en) * | 2018-06-19 | 2022-01-14 | Армасьютика Инк. | Bifunctional compositions for cancer treatment |
| WO2021105761A1 (en) * | 2019-11-29 | 2021-06-03 | Institut National De La Santé Et De La Recherche Médicale (Inserm) | Compositions and their uses for treating cancers |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20020011528A (en) | 2002-02-09 |
| EP1313475A1 (en) | 2003-05-28 |
| AU2001275821A1 (en) | 2002-02-25 |
| KR100390332B1 (en) | 2003-07-07 |
| WO2002013826A9 (en) | 2003-11-13 |
| EP1313475A4 (en) | 2004-08-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6949510B2 (en) | Uses of diterpenoid triepoxides as an anti-proliferative agent | |
| AU2005259002B2 (en) | Treatment of cancer | |
| Khalife et al. | Thymoquinone from Nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro | |
| Lian et al. | Genistein‐induced G2‐M arrest, p21WAF1 upregulation, and apoptosis in a non‐small‐cell lung cancer cell line | |
| KR0171893B1 (en) | Compounds for the reversal of multidrug resistance of cancer cells against cytotoxic drugs and pharmaceutical compositions containing them | |
| Sugiyama et al. | Enhancing effects of green tea components on the antitumor activity of adriamycin against M5076 ovarian sarcoma | |
| EP1313475A1 (en) | Anti-cancer composition composed of anti-cancer and anti-malarial drugs | |
| US20030114393A1 (en) | Use of steroidal alkaloids to reverse multidrug resistance | |
| EP1901750A2 (en) | Tumor treatment with gliotoxin derivatives | |
| Shi et al. | Reversal effect of tyroservatide (YSV) tripeptide on multi-drug resistance in resistant human hepatocellular carcinoma cell line BEL-7402/5-FU | |
| Song et al. | Magnolin targeting of ERK1/2 inhibits cell proliferation and colony growth by induction of cellular senescence in ovarian cancer cells | |
| Zhong et al. | The regulatory roles of calcium channels in tumors | |
| AU2005211937B2 (en) | Methods for treating resistant or refractory tumors | |
| EP1289513B1 (en) | Use of aloe-emodin in the treatment of neuroectodermal tumors | |
| TW201249453A (en) | Berberine-containing pharmaceutical composition for inhibiting cancer stem cells growth or carcinoma metastasis and application thereof | |
| US20240042040A1 (en) | Cisplatin analogue with potent anti-cancer effects and synthesis thereof | |
| CN112294829A (en) | Application of salidroside in preparation of medicine for treating or preventing cancer | |
| 권혜정 | Targeting XIAP in mucoepidermoid carcinoma of salivary gland: A novel therapeutic strategy of nitidine chloride | |
| Park et al. | Compound 3K, a specific pyruvate kinase M2 inhibitor, induces autophagic cell death through the disruption of the glycolytic pathway in ovarian cancer cells | |
| CN111689870A (en) | Honokiol-chlorambucil co-prodrug with lymphocyte leukemia resisting effect and preparation method and application thereof | |
| Engi | Perspectives in cancer chemotherapy, in vitro and in vivo experiments | |
| NZ552508A (en) | Treatment of cancer with pharmaceutical agents together with anti-Hsp 90 antibodies | |
| Gerrard | Modulation of P-glycoprotein by Zosuquidar Trihydrochloride | |
| US20060293388A1 (en) | Pomolic acid, its isomers, derivatives and their uses, pharmaceutical composition, method to prepare the pharmaceutical composition and method for treating multidrug resistant tumours |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2001953364 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2001953364 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| COP | Corrected version of pamphlet |
Free format text: INTERNATIONAL SEARCH REPORT ADDED (1 PAGE) |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |